Abstract
Aims
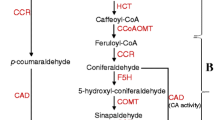
Induction of lignin biosynthesis is an adaptive response of plants subjected to many abiotic stresses. In this study, we examined the response of lignin biosynthesis to copper (Cu) stress, with a particular focus on the regulatory mechanism.
Methods
We performed a transcriptomic analysis of rice (Oryza sativa L.) roots, and the microarray data on lignin biosynthesis pathway genes were corroborated by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis. Physiological analyses of rice seedlings treated with Cu(II) sulfate (CuSO4) were used to confirm the relationship between excess Cu and lignin biosynthesis. In addition, we examined the role of hydrogen peroxide (H2O2) in Cu-induced lignin biosynthesis through pretreatments with an NADPH oxidase inhibitor (diphenyleneiodonium, DPI) and a H2O2 scavenger (dimethylthiourea, DMTU).
Results
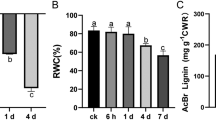
Lignin biosynthesis pathway genes were upregulated under Cu stress. The lignin content of rice roots increased significantly with increasing concentrations and durations of Cu treatment; elevations in root lignin content were correlated with marked inhibitions in root growth. Pretreatments with DPI and DMTU inhibited the activities of Cu-induced lignin polymerization enzymes (peroxidase, POD and laccase, LAC) and lignin accumulation in rice roots. Conversely, exogenous H2O2 increased the root lignin content.
Conclusions
Rice roots under Cu stress accumulate lignin through enhanced polymerization of lignin monolignol, a mechanism that requires Cu stress induced H2O2.







Similar content being viewed by others
References
Ali MB, Singh N, Shohael AM, Hahn EJ, Paek KY (2006) Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci 171(1):147–154
Berthet S, Demont–Caulet N, Pollet B, Bidzinskia P, Cézarda L, Le Brisa P, Borrega N, Herve J, Blondet E, Balzergue S, Lapierre C, Jouanin L (2011) Disruption of LACCASE4 and 17 results in tissue–specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 23(3):1124–1137
Bhargava A, Clabaugh I, To JP, Maxwell BB, Chiang YH, Schaller GE, Loraine A, Kieber JJ (2013) Identification of cytokinin-responsive genes using microarray meta–analysis and RNA-Seq in Arabidopsis. Plant Physiol 162(1):272–29
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Bonawitz ND, Chapple C (2010) The genetics of lignin biosynthesis: connecting genotype to phenotype. Annu Rev Genet 44:337–363
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72(1):248–254
Bykov I (2008) Characterization of natural and technical lignins using FTIR spectroscopy. Dissertation, Lulea University of Technology
Chaoui A, Jarrar B, EL Ferjani E (2004) Effects of cadmium and copper on peroxidase, NADH oxidase and IAA oxidase activities in cell wall, soluble and microsomal membrane fractions of pea roots. J Plant Physiol 161(11):1225–1234
Chen EL, Chen YA, Chen LM, Liu ZH (2002) Effect of copper on peroxidase activity and lignin content in Raphanus sativus. Plant Physiol Bioch 40:439–444
Cheng H, Tam NFY, WangY LS, Chen G, Ye Z (2012) Effects of copper on growth, radial oxygen loss and root permeability of seedlings of the mangroves Bruguiera gymnorrhiza and Rhizophora stylosa. Plant Soil 359:255–266
Dai D, Fan M (2011) Investigation of the dislocation of natural fibres by Fourier-transform infrared spectroscopy. VibSpectrosc 55(2):300–306
De Jaegher G, Boyer N, Gaspar T (1985) Thigmomorphogenesis in Bryoniadioica: Changes in soluble and wall peroxidases, phenylalanine ammonia-lyase activity, cellulose, lignin content and monomeric constituents. Plant Growth Regul 3(2):133–148
Diaz J, Bernal A, Pomar F, Merino F (2001) Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci 161:179–188
Dunand C, Crèvecoeur M, Penell C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174(2):332–341
Ederli L, Reale L, Ferranti F, Pasqualini S (2004) Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol Plant 121:66–74
Fecht-Christoffers MM, Führs H, Braun HP, Horst WJ (2006) The role of hydrogen peroxide–producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiol 140(4):1451–1463
Fukuda H, Komamine A (1982) Lignin synthesis and its related enzymes as markers of tracheary-element differentiation in single cells isolated from the mesophyll of Zinnia elegans. Planta 155(5):423–430
Gao L, Peng KJ, Chen YH, Wang GP, Shen ZG (2012) Roles of apoplastic peroxidases, laccases, and lignification in the manganese tolerance of hyperaccumulator Phytolacca americana. Acta Physiol Plant 34(1):151–159
Hammond-Kosack KE, Jones JD (1996) Resistance gene-dependent plant defense responses. Plant Cell 8(10):1773–1791
Herrero J, Carrasco AE, Zapata JM (2013) Looking for Arabidopsis thaliana peroxidases involved in lignin biosynthesis. Plant Physiol Bioch 67:77–86
Hopmans P (1990) Stem deformity in Pinus radiata plantations in south-eastern Australia. Plant Soil 122(1):97–104
Hossain MA, Hossain AZ, Kihara T, Koyama H, Hara T (2005) Aluminum-induced lipid peroxidation and lignin deposition are associated with an increase in H2O2 generation in wheat seedlings. Soil Sci Plant Nutr 51(2):223–230
Iiyama K, Wallis AFA (1988) An improved acetyl bromide procedure for determining lignin in woods and wood pulps. Wood Sci Technol 22(3):271–280
Iiyama K, Wallis AFA (1990) Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agr 51(2):145–161
Jaleel CA, Manivannan P, Sankar B, Kishorekumar A, Gopi R, Somasundaram R, Panneerselvam R (2007) Water deficit stress mitigation by calcium chloride in Catharanthu sroseus: Effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf B: Biointerfaces 60(1):110–116
Kärkönen A, Koutaniemi S (2010) Lignin biosynthesis studies in plant tissue cultures. J Integr Plant Biol 52(2):176–185
Kováčik J, Bačkor M (2007) Phenylalanine ammonia-lyase and phenolic compounds in chamomile tolerance to cadmium and copper excess. Water Air Soil Poll 185(1–4):185–193
Kováčik J, Klejdus B (2008) Dynamics of phenolic acids and lignin accumulation in metal-treated Matricaria chamomilla roots. Plant Cell Rep 27(3):605–615
Kováčik J, Grúz J, Klejdus B, Štork F, Marchiosid R, Ferrarese-Filhod O (2010) Lignification and related parameters in copper-exposed Matricaria chamomilla roots: Role of H2O2 and NO in this process. Plant Sci 179(4):383–389
Kováčik J, Klejdus B, Hedbavny J, Zoń J (2011) Significance of phenols in cadmium and nickel uptake. J Plant Physiol 168(6):576–584
Larkindale J, Huang B (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide and ethylene. J Plant Physiol 161(4):405–413
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Bioch 48(8):673–682
Li Y, Qian Q, Zhou Y, Yan M, Sun L, Zhang M, Fu Z, Wang Y, Han B, Pang X, Chen M, Li J (2003) BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell 15(9):2020–2031
Lin CC, Kao CH (2001) Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci 160(2):323–329
Lin CC, Chen LM, Liu ZH (2005) Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci 168(3):855–861
Lin CY, Trinh NN, Fu SF, Hsiung YC, Chia LC, Lin CW, Huang HJ (2013) Comparison of early transcriptome responses to copper and cadmium in rice roots. Plant Mol Biol 81(4–5):507–522
Marschner H (2012) Mineral nutrition of higher plants, 3rd edn. Academic, London, p 651
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve-Zacheo T (2006) Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol 170(3):501–512
Memon AR, Schröder P (2009) Implications of metal accumulation mechanisms to phytoremediation. Environ Sci Pollut Res Int 16(2):162–175
Mika A, Luthje S (2003) Properties of guaiacol peroxidase activities isolated from corn root plasma membranes. Plant Physiol 132:1489–1498
Moura JCMS, Bonine CAV, De Oliveira FVJ, Dornelas MC, Mazzafera P (2010) Abiotic and biotic stresses and changes in the lignin content and composition in plants. J Integr Plant Biol 52(4):360–376
Navari-Izzo F, Quartacci MF (2001) Phytoremediation of metals: tolerance mechanisms against oxidative stress. Minerva Biotechnol 13:23–83
Ogo Y, Kakei Y, Itai RN, Kobayashi T, Nakanishi H, Takahashi H, Nakazono M, Nishizawa NK (2014) Spatial transcriptomes of iron-deficient and cadmium-stressed rice. New Phytol 201(3):781–794
Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406(6797):731–734
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway spruce (Piceaabies L.). Plant Physiol 106(1):53–60
Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A (1999) The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol 119(3):849–858
Sasaki M, Yamamoto Y, Matsumoto H (1996) Lignin deposition induced by aluminum in wheat (Triticumaestivum) roots. Physiol Plant 96(2):193–198
Schmidt R, Mieulet D, Hubberten HM, Obata T, Hoefgen R, Fernie AR, Fisahn J, San Segundo B, Guiderdoni E, Schippers JHM, Mueller-Roeber B (2013) SALT-RESPONSIVE ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 25(6):2115–2131
Sgherri C, Stevanovic B, Navari-Izzo F (2004) Role of phenolics in the antioxidative status of the resurrection plant Ramonda serbica during dehydration and rehydration. Physiol Plant 122:478–485
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16(3):144–158
Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128(4):1271–1281
Van Acker R, Vanholme R, Storme V, Mortimer JC, Dupree P, Boerjan W (2013) Lignin biosynthesis perturbations affect secondary cell wall composition and saccharification yield in Arabidopsis thaliana. Biotechnol Biofuels 6:46
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153(3):895–905
Wang GD, Li QJ, Luo B, Chen XY (2004) Ex planta phytoremediation of trichlorophenol and phenolic allelochemicals via an engineered secretory laccase. Nat Biotechnol 22:893–897
Wang J, Wang C, Zhu M, Yu Y, Zhang Y, Wei Z (2008) Generation and characterization of transgenic poplar plants overexpressing a cotton laccase gene. Plant Cell Tiss Org 93(3):303–310
Yan C, Yin M, Zhang N, Jin Q, Fang Z, Lin Y, Cai Y (2014) Stone cell distribution and lignin structure in various pear varieties. SciHortic-Amsterdam 174:142–150
Yang YJ, Cheng LM, Liu ZH (2007) Rapid effect of cadmium on lignin biosynthesis in soybean roots. Plant Sci 172(3):632–639
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430
Zhao Q, Nakashima J, Chen F, Yin Y, Fu C, Yun J, Shao H, Wang X, Wang Z-Y, Dixon RA (2013) LACCASE is necessary and nonredundant with PEROXIDASE for lignin polymerization during vascular development in Arabidopsis. Plant Cell 25(10):3976–3987
Zheng X, Van Huystee RB (1992) Peroxidase-regulated elongation of segments from peanut hypocotyls. Plant Sci 81(1):47–56
Ziva R, Spela B, Ana R, Matej K, Igor M, Bjorn U, Kristina G (2014) GoMapMan: integration, consolidation and visualization of plant gene annotations within the MapMan ontology. Nucleic Acids Res 42(D1):D1167–D1175
Acknowledgments
This work was supported by research grants from the Project of the National Natural Science Foundation of China (No. 31172021),“the Fundamental Research Funds for the Central Universities (KYRC201302, KYTZ201402)” and the Innovative Research Team Development Plan of the Ministry of Education of China (grant no. IRT1256).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Henk Schat.
Rights and permissions
About this article
Cite this article
Liu, Q., Zheng, L., He, F. et al. Transcriptional and physiological analyses identify a regulatory role for hydrogen peroxide in the lignin biosynthesis of copper-stressed rice roots. Plant Soil 387, 323–336 (2015). https://doi.org/10.1007/s11104-014-2290-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2290-7