Abstract
Aims
Rice fields are an important source for the greenhouse gas methane. Plants play an essential role in carbon supply for soil microbiota, but the influence of the microbial community on carbon cycling is not well understood.
Methods
Microcosms were prepared using sand-vermiculite amended with different soils and sediments, and planted with rice. The microcosms at different growth stages were pulse-labeled with 13CO2 followed by tracing 13C in plant, soil and atmospheric carbon pools and quantifying the abundance of methanogenic archaea in rhizosphere soil.
Results
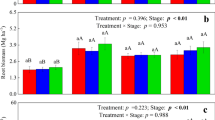
Overall, >85 % of the freshly assimilated carbon was allocated in aboveground plant biomass, approximately 10 % was translocated into the roots and < 2 % was recovered in soil organic matter, independently from soil type. Only about 0.3 % was transformed to CH4, but emission of 13C-labeled CH4 started immediately and 13C enrichment revealed that plant-derived carbon was an important source for methanogenesis. The results further demonstrated that carbon assimilation and translocation processes, microbial abundance and gas emission were not only affected by the plant growth stage, but also by the content and type of soil in which the rice plants grew.
Conclusions
The study illustrates the close ties between plant physiology, soil properties and microbial communities for carbon turnover and ecosystem functioning.






Similar content being viewed by others
References
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001a) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3:139–148
Aulakh MS, Wassmann R, Bueno C, Rennenberg H (2001b) Impact of root exudates of different cultivars and plant development stages of rice (Oryza sativa L.) on methane production in a paddy soil. Plant Soil 230:77–86
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere [Review]. FEMS Microbiol Ecol 68:1–13
Brand WA (1996) High precision isotope ratio monitoring techniques in mass spectrometry. J Mass Spectrom 31:225–235
Brüggemann N, Gessler A, Kayler Z, Keel SG, Badeck F, Barthel M, Boeckx P, Buchmann N, Brugnoli E, Esperschütz J, Gavrichkova O, Ghashghaie J, Gomez-Casanovas N, Keitel C, Knohl A, Kuptz D, Palacio S, Salmon Y, Uchida Y, Bahn M (2011) Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences 8:3457–3489
Burggraf S, Huber H, Stetter KO (1997) Reclassification of the crenarchaeal orders and families in accordance with 16S rRNA sequence data. Int J Syst Bacteriol 47:657–660
Chanton JP, Bauer JE, Glaser PA, Siegel DI, Kelley CA, Tyler SC, Romanowicz EH, Lazrus A (1995) Radiocarbon evidence for the substrates supporting methane formation within northern Minnesota peatlands. Geochim Cosmochim Acta 59:3663–3668
Chanton JP, Whiting GJ, Blair NE, Lindau CW, Bollich PK (1997) Methane emission from rice: stable isotopes, diurnal variations, and CO2 exchange. Glob Biogeochem Cycles 11:15–27
Clode PL, Kilburn MR, Jones DL, Stockdale EA, Cliff JB III, Herrmann AM, Murphy DV (2009) In situ mapping of nutrient uptake in the rhizosphere using nanoscale secondary ion mass spectrometry. Plant Physiol 151:1751–1757
Conrad R (2007) Microbial ecology of methanogens and methanotrophs. Adv Agron 96:1–63
Dannenberg S, Conrad R (1999) Effect of rice plants on methane production and rhizospheric metabolism in paddy soil. Biogeochem 45:53–71
Ding WX, Cai ZC, Tsuruta H, Li XP (2003) Key factors affecting spatial variation of methane emissions from freshwater marshes. Chemosphere 51:167–173
Dorodnikov M, Knorr K, Kuzyakov Y, Wilmking M (2011) Plant-mediated CH4 transport and contribution of photosynthates to methanogenesis at a boreal mire: a 14C pulse-labeling study. Biogeosciences 8:2365–2375
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631
Fischer H, Eckhardt KU, Meyer A, Neumann G, Leinweber P, Fischer K, Kuzyakou Y (2010) Rhizodeposition of maize: short-term carbon budget and composition. J Plant Nutr Soil Sci 173:67–79
Ge T, Yuan H, Zhu H, Wu X, Nie S, Liu C, Tong C, Wu J, Brookes P (2012a) Biological carbon assimilation and dynamics in a flooded rice - soil system. Soil Biol Biochem 48:39–46
Ge ZM, Zhou X, Kellomäki S, Biasi C, Wang KY, Peltola H, Martikainen PJ (2012b) Carbon assimilation and allocation (13C labeling) in a boreal perennial grass (Phalaris arundinacea) subjected to elevated temperature and CO2 through a growing season. Environ Exp Bot 75:150–158
Grosskopf R, Janssen PH, Liesack W (1998) Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol 64:960–969
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-induced pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stat Circ 347:1–32
Holzapfel-Pschorn A, Conrad R, Seiler W (1985) Production, oxidation and emission of methane in rice paddies. FEMS Microbiol Ecol 31:343–351
Joabsson A, Christensen TR (2001) Methane emissions from wetlands and their relationship with vascular plants: an Arctic example. Glob Chang Biol 7:919–932
Kankaala P, Käki T, Mäkelä S, Ojala A, Pajunen H, Arvola L (2005) Methane efflux in relation to plant biomass and sediment characteristics in stands of three common emergent macrophytes in boreal mesoeutrophic lakes. Glob Chang Biol 11:145–153
Ke X, Lu Y, Conrad R (2014) Different behavior of methanogenic archaea and Thaumarchaeota in rice field microcosms. FEMS Microbiol Ecol 87:18–29
Keppler F, Hamilton JTG, Brass M, Röckmann T (2006) Methane emissions from terrestrial plants under aerobic conditions. Nature 439:187–191
Kimura M, Murase J, Lu YH (2004) Carbon cycling in rice field ecosystems in the context of input, decomposition and translocation of organic materials and the fates of their end products (CO2 and CH4) [Review]. Soil Biol Biochem 36:1399–1416
King JY, Reeburgh WS (2002) A pulse-labeling experiment to determine the contribution of recent plant photosynthates to net methane emission in arctic wet sedge tundra. Soil Biol Biochem 34:173–180
King JY, Reeburgh WS, Thieler KK, Kling GW, Loya WM, Johnson LC, Nadelhoffer KJ (2002) Pulse-labeling studies of carbon cycling in Arctic tundra ecosystems: The contribution of photosynthates to methane emission. Global Biogeochem Cycles 16: 1062-doi:10.1029/2001GB001456
Krüger M, Frenzel P, Kemnitz D, Conrad R (2005) Activity, structure and dynamics of the methanogenic archaeal community in a flooded Italian rice field. FEMS Microbiol Ecol 51:323–331
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review J Plant Nutr Soil Sci 163:421–431
Kuzyakov Y, Ehrensberger H, Stahr K (2001) Carbon partitioning and below-ground translocation by Lolium perenne. Soil Biol Biochem 33:61–74
Kuzyakov Y, Gavrichkova O (2010) Time lag between photosynthesis and carbon dioxide efflux from soil: a review of mechanisms and controls [review]. Glob Chang Biol 16:3386–3406
Lehner B, Döll P (2004) Development and validation of a global database of lakes, reservoirs and wetlands. J Hydrol 296:1–22
LeMer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: A review [Review]. Eur J Soil Biol 37:25–50
Liesack W, Schnell S, Revsbech NP (2000) Microbiology of flooded rice paddies [Review]. FEMS Microbiol Rev 24:625–645
Lowe DC (2006) A green source of surprise. Nature 439:148–149
Lu YH, Watanabe A, Kimura M (2002) Input and distribution of photosynthesized carbon in a flooded rice soil. Global Biogeochem Cycles 16: 1085-doi:10.1029/2002GB001864
Lueders T, Manefield M, Friedrich MW (2004) Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ Microbiol 6:73–78
Lynch JM, Whipps JM (1990) Substrate Flow in the Rhizosphere. Plant Soil 129:1–10
Marx M, Buegger F, Gattinger A, Marschner B, Zsolnay A, Munch JC (2007) Determination of the fate of 13C labelled maize and wheat rhizodeposit-C in two agricultural soils in a greenhouse experiment under 13C-CO2-enriched atmosphere. Soil Biol Biochem 39:3043–3055
Megonigal JP, Hines ME, Visscher PT (2004) Anaerobic metabolism: linkages to trace gases and aerobic processes. In: Schlesinger WH, Holland HD, Turekian KK (eds) Treatise on Geochemistry, vol 8, Biogeochemistry. Elsevier-Pergamon, Oxford, pp 317–424
Meharg AA, Killham K (1991) A novel method of quantifying root exudation in the presence of soil microflora. Plant Soil 133:111–116
Minoda T, Kimura M (1994) Contribution of photosynthesized carbon to the methane emitted from paddy fields. Geophys Res Lett 21:2007–2010
Minoda T, Kimura M, Wada E (1996) Photosynthates as dominant source of CH4 and CO2 in soil water and CH4 emitted to the atmosphere from paddy fields. J Geophys Res 101:21091–21097
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and control. Agronomie 23:375–396
Nguyen NV, Ferrero A (2006) Meeting the challenges of global rice production. Paddy Water Environ 4:1–9
Ostle N, Ineson P, Benham D, Sleep D (2000) Carbon assimilation and turnover in grassland vegetation using an in situ 13CO2 pulse labelling system. Rapid Commun Mass Spec 14:1345–1350
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
R Development Core Team R (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramakrishnan B, Lueders T, Conrad R, Friedrich M (2000) Effect of soil aggregate size on methanogenesis and archaeal community structure in anoxic rice field soil. FEMS Microbiol Ecol 32:261–270
Rice AL, Gotoh AA, Ajie HO, Tyler SC (2001) High-precision continuous-flow measurement of d13C and dD of atmospheric CH4. Anal Chem 73:4104–4110
Sass RL, Fisher FM, Harcombe PA, Turner FT (1990) Methane production and emission in a Texas rice field. Glob Biogeochem Cycles 4:47–68
Sass RL, Fisher FM, Huang Y (2000) A process-based model for methane emissions from irrigated rice fields: Experimental basis and assumptions. Nutr Cycl Agroecosyst 58:249–258
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Molec Biol Rev 61:262–280
Schinner F, Öhlinger R, Kandeler E, Margesin R (1996) Methods in soil chemistry. Methods in Soil Biology. Springer, London, pp 397–416
Schütz H, Holzapfel-Pschorn A, Conrad R, Rennenberg H, Seiler W (1989) A 3-year continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian rice paddy. J Geophys Res 94:16405–16416
Sessitsch A, Weilharter A, Gerzabek MH, Kirchmann H, Kandeler E (2001) Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl Environ Microbiol 67:4215–4224
Steinberg LM, Regan JM (2008) Phylogenetic comparison of the methanogenic communities from an acidic, oligotrophic fen and an anaerobic digester treating municipal wastewater sludge. Appl Environ Microbiol 74:6663–6671
Tokida T, Adachi M, Cheng W, Nakajima Y, Fumoto T, Matsushima M, Nakamura H, Okada M, Sameshima R, Hasegawa T (2011) Methane and soil CO2 production from current-season photosynthates in a rice paddy exposed to elevated CO2 concentration and soil temperature. Glob Chang Biol 17:3327–3337
Vann CD, Megonigal JP (2003) Elevated CO2 and water depth regulation of methane emissions: Comparison of woody and non-woody wetland plant species. Biogeochem 63:117–134
Wardle DA, Bardgett RD, Klironomos JN, Setala H, VanderPutten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota [Review]. Science 304:1629–1633
Warembourg FR, Estelrich HD (2001) Plant phenology and soil fertility effects on belowground carbon allocation for an annual (Bromus madritensis) and a perannial (Bromus erectus) grass species. Soil Biol Biochem 33:1291–1303
Watanabe A, Machida N, Takahashi K, Kitamura S, Kimura M (2004) Flow of photosynthesized carbon from rice plants into the paddy soil ecosystem at different stages of rice growth. Plant Soil 258:151–160
White JR, Shannon RD, Weltzin JF, Pastor J, Bridgham SD (2008) Effects of soil warming and drying on methane cycling in a northern peatland mesocosm study. J Geophys Res -Biogeosci 113: G00A06-doi:10.1029/2007JG000609
Whiting GJ, Chanton JP (1993) Primary production control of methane emission from wetlands. Nature 364:794–795
Wu WX, Liu W, Lu HH, Chen YX, Devare M, Thies J (2009) Use of 13C labeling to assess carbon partitioning in transgenic and nontransgenic (parental) rice and their rhizosphere soil microbial communities. FEMS Microbiol Ecol 67:93–102
Wu Y, Tan H, Deng Y, Wu J, Xu X, Wang Y, Tang Y, Higashi T, Cui X (2010) Partitioning pattern of carbon flux in Kobresia grassland on the Qinghai-Tibetan Plateau revealed by field 13C pulse-labeling. Glob Chang Biol 16:2322–2333
Yuan Q, Pump J, Conrad R (2012) Partitioning of CH4 and CO2 production originating from rice straw, soil and root organic carbon in rice microcosms. Plos One 7: e49073-doi:10.1371/journal.pone.0049073
Acknowledgments
We thank Dr. Jennifer Pratscher (University of Warwick, Coventry, United Kingdom) for taking part in qPCR analysis and Philip Weyrauch (University of Münster, Germany) for performing gas measurements during soil incubation experiments. We are also grateful to Dr. Elisabetta Lupotto (CRA-RIS, Vercelli, Italy) for providing rice seeds, Prof. Dr. Peter Frenzel (Max Planck Institute for Terrestrial Microbiology, Marburg, Germany) for supplying Chinese paddy soil, and Dr. Quan Yuan (Max Planck Institute for Terrestrial Microbiology, Marburg, Germany) for critically reading of the manuscript. This research was supported by the Research Center for Synthetic Microbiology (‘Synmikro’) of the Landes-Offensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz (LOEWE) and the Max Planck Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Paul Bodelier.
Rights and permissions
About this article
Cite this article
Pump, J., Conrad, R. Rice biomass production and carbon cycling in 13CO2 pulse-labeled microcosms with different soils under submerged conditions. Plant Soil 384, 213–229 (2014). https://doi.org/10.1007/s11104-014-2201-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2201-y