Abstract
Background and aims
Conversion of natural forests to plantations often results in a considerable loss of plant species and thus likely a reduction in quantity and quality of plant debris entering the soil. Larch plantation is widespread in northeastern China, but its ecological impacts receive little attention. This study aimed to assess soil quality under larch stands against the secondary forest stands using a suite of soil chemical and microbiological properties.
Methods
Four pairs of larch plantations and secondary forests were randomly selected from a mountainous area and mineral soils of top 15 cm were collected from each field.
Results
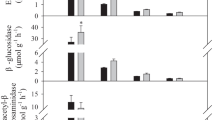
Soil carbon (C) and nitrogen (N) concentrations, microbial biomass, C and N mineralization and the activities of hydrolytic enzymes were significantly lower in the larch plantations than those in the secondary forests. However, light fraction C as a proportion of soil C was greater in the larch plantations, suggesting less accumulation and stabilization of soil C to heavy fraction in the larch plantations compared to the secondary forests. We also used δ15N records in light and heavy fractions to derive the relative stability of soil C and found that soil C stability was lower in the larch plantations. This was supported by Fourier transform infrared spectroscopy analysis because carboxylate stretching, which might result from microbial oxidation, was less abundant in the larch plantations.
Conclusions
The differences in soil organic matter quality between the larch plantations and the secondary forests were reliably reflected in soil microbial properties and microbially-mediated processes. Our results indicated that the larch plantations reduced soil quality as well as nutrient cycling rate.




Similar content being viewed by others
Abbreviations
- ESOC:
-
Extractable soil organic carbon
- FTIR:
-
Fourier transform infrared spectroscopy
- LP:
-
Larch plantation
- MBC:
-
Microbial biomass carbon
- MBN:
-
Microbial biomass nitrogen
- SF:
-
Secondary forest
- SOC:
-
Soil organic carbon
- SOM:
-
Soil organic matter
References
Adams MA, Grierson PF (2001) Stable isotopes at natural abundance in terrestrial plant ecology and ecophysiology: an update. Plant Biol 3:299–310
Arai H, Tokuchi N (2010) Soil organic carbon accumulation following afforestation in a Japanese coniferous plantation based on particle-size fractionation and stable isotope analysis. Geoderma 159:425–430
Boone RD (1994) Light-fraction soil organic matter: origin and contribution to net nitrogen mineralization. Soil Biol Biochem 26:1459–1468
Boström B, Comstedt D, Ekblad A (2007) Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 153:89–98
Brassard BW, Chen HYH, Bergeron Y, Paré D (2011) Differences in fine root productivity between mixed- and single-species stands. Funct Ecol 25:238–246
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and release of soil N: a rapid direct extraction method to measure microbial biomass N in soil. Soil Biol Biochem 17:837–842
Chen DK, Zhou XF, Zhu N (1994) Natural secondary forest—structure, function, dynamics and management. Northeast Forestry University Press, Haerbin
Conen F, Zimmermann M, Leifeld J, Seth B, Alewell C (2007) Relative stability of soil carbon revealed by shifts in δ15N and C:N ratio. Biogeosci Discuss 4:2915–2928
Cuevas E, Brown S, Lugo AE (1991) Above- and belowground organic matter storage and production in a tropical pine plantation and a paired broadleaf secondary forest. Plant Soil 135:257–268
Das SK, Varma A (2011) Role of enzymes in maintaining soil health. In: Shukla G, Varma A (eds) Soil Enzymology. Springer-Verla, Berlin, pp 43–60
Dijkstra P, Ishizu A, Doucett R, Hart SC, Schwartz E, Menyailo OV, Hungate BA (2006) 13C and 15N natural abundance of the soil microbial biomass. Soil Biol Biochem 38:3257–3266
Doran JW (2002) Soil health and global sustainability: translating science into practice. Agr Ecosyst Environ 88:119–127
Doran JW, Zeiss MR (2000) Soil health and sustainability: managing the biotic component of soil quality. Appl Soil Ecol 15:3–11
Ehleringer JR, Buchmann N, Flanagan LB (2000) Carbon isotope ratios in belowground carbon cycle processes. Ecol Appl 10:412–422
Feldpausch TR, Rondon MA, Fernandes ECM, Riha SJ, Wandelli E (2004) Carbon and nutrient accumulation in secondary forests regenerating from pastures in central Amazônia. Ecol Appl 14:164–176
Feng X (2002) A theoretical analysis of carbon isotope evolution of decomposing plant litters and soil organic matter. Global Biogeochem Cycles 16:1119–1130
Guo LB, Gifford RM (2002) Soil carbon stocks and land use change: a meta analysis. Global Change Biol 8:345–360
Haile-Mariam S, Collins HP, Wright S, Paul EA (2008) Fractionation and long-term laboratory incubation to measure soil organic matter dynamics. Soil Sci Soc Am J 72:370–378
Hassett JE, Zak DR (2005) Aspen harvest intensity decreases microbial biomass, extracellular enzyme activity, and soil nitrogen cycling. Soil Sci Soc Am J 69:227–235
Hobbie EA, Ouimette AP (2009) Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95:355–371
Högberg P (1997) 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R (2007) How strongly can forest management influence soil carbon sequestration? Geoderma 137:253–268
Johnston CT, Aochi YO (1996) Fourier transform infrared and Raman spectroscopy. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. Part 3. Chemical methods. Soil Science Society of America, Madison, pp 269–321
Kasel S, Bennett LT (2007) Land-use history, forest conversion, and soil organic carbon in pine plantations and native forests of south eastern Australia. Geoderma 137:401–413
Li Y, Xu M, Zou XM, Shi PJ, Zhang YQ (2005) Comparing soil organic carbon dynamics in plantation and secondary forest in wet tropics in Puerto Rico. Global Change Biol 11:239–248
Liao CZ, Luo YQ, Fang CM, Li B (2010) Ecosystem carbon stock influenced by plantation practice: implications for planting forests as a measure of climate change mitigation. PLoS One 5:e10867
Liu SR, Li XM, Niu LM (1998) The degradation of soil fertility in pure larch plantations in the northeastern part of China. Ecol Eng 10:75–86
Lugo AE (1992) Comparison of tropical tree plantations with secondary forests of similar age. Ecol Monogr 62:1–41
Malchair S, Carnol M (2009) Microbial biomass and C and N transformations in forest floors under European beech, sessile oak, Norway spruce and Douglas-fir at four temperate forest sites. Soil Biol Biochem 41:831–839
Menyailo OV, Hungate BA, Zech W (2002) The effect of single tree species on soil microbial activities related to C and N cycling in the Siberian artificial afforestation experiment. Plant Soil 242:183–196
Nambu K, van Hees PAW, Jones DL, Vinogradoff S, Lundström US (2008) Composition of organic solutes and respiration in soils derived from alkaline and non-alkaline parent materials. Geoderma 144:468–477
Parham JA, Deng SP (2000) Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol Biochem 32:1183–1190
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Schoenholtz SH, Van Miegroet H, Burger JA (2000) A review of chemical and physical properties as indicators of forest soil quality: challenges and opportunities. For Ecol Manage 138:335–356
Schulp CJE, Nabuurs G, Verburg PH, de Waal RW (2008) Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. For Ecol Manage 256:482–490
Seely B, Welham C, Blanco JA (2010) Towards the application of soil organic matter as an indicator of forest ecosystem productivity: Deriving thresholds, developing monitoring systems, and evaluating practices. Ecol Indic 10:999–1008
Shi W (2011) Agricultural and ecological significance of soil enzymes: soil carbon sequestration and nutrient cycling. In: Shukla G, Varma A (eds) Soil Enzymology. Springer-Verla, Berlin, pp 43–60
Shi W, Dell E, Bowman D, Iyyemperumal K (2006) Soil enzyme activities and organic matter composition in a turfgrass chronosequence. Plant Soil 288:285–296
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Causack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Strickland TC, Sollins P (1987) Improved method for separating light and heavy fraction organic material from soil. Soil Sci Soc Am J 51:1390–1393
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vesterdal L, Schmidt IK, Callesen I, Nilsson LO, Gundersen P (2008) Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For Ecol Manage 255:35–48
von Mersi W, Schinner F (1996) Dehydrogenase activity with the substrate INT. In: Schinner F, Õhlinger R, Kandeler E, Margesin R (eds) Methods in Soil Biology. Springer, Berlin, pp 243–245
Wardle DA (1998) Controls of temporal variability of the soil microbial biomass: A global-scale synthesis. Soil Biol Biochem 30:1627–1637
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Yang K, Zhu JJ, Zhang M, Yan QL, Sun OJ (2010) Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J Plant Ecol 3:175–182
Yang K, Zhu JJ, Yan QL, Zhang JX (2012) Soil enzyme activities as potential indicators of soluble organic nitrogen pools in forest ecosystems of Northeast China. Ann For Sci 69:795–803
Yao HY, Shi W (2010) Soil organic matter stabilization in turfgrass ecosystems: Importance of microbial processing. Soil Biol Biochem 42:642–648
Zhang QZ, Wang CK, Wang XC, Quan XK (2009) Carbon concentration variability of 10 Chinese temperate tree species. For Ecol Manage 258:722–727
Zhu JJ, Mao ZH, Hu LL, Zhang JX (2007) Plant diversity of secondary forests in response to anthropogenic disturbance levels in montane regions of northeastern China. J Forest Res 12:403–416
Acknowledgments
This study was financially supported by the National Basic Research Program of China (2012CB416906, 2011CB403205), and the CAS/SAFEA International Partnership Program for Creative Research Teams (KZCX2-YW-445). We appreciate Ms. Shuang Xu for analyzing soil carbon and nitrogen mineralization.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Per Ambus.
Rights and permissions
About this article
Cite this article
Yang, K., Shi, W. & Zhu, JJ. The impact of secondary forests conversion into larch plantations on soil chemical and microbiological properties. Plant Soil 368, 535–546 (2013). https://doi.org/10.1007/s11104-012-1535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1535-6