Abstract
Background and aims
Below-ground grass competition limits woody establishment in savannas. N2-fixing legumes may, however, have a nutritional advantage over broad-leaved species. We hypothesised that broad-leaved non-legume savanna thicket species would be more severely constrained by grass competition for N and consequently respond more to N-fertilization than the legume, Acacia karroo.
Methods
A. karroo and five non-legume thicket species (Maytenus senegalensis, M. heterophylla, Euclea divinorum, Ziziphus mucronata, Schotia brachypetala) were grown together in an irrigated competition experiment with clipped-, unclipped-grass and without grass with/without N-fertilizer. The biomass, foliar nutrient, δ13C and δ15N of grasses and woody species were determined.
Results
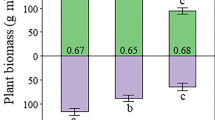
Growth of both A. karroo and the non-legume species was equally sensitive (c. 90 % reduction) to both clipped- and unclipped-grass competition, regardless of N-fertilization. With grass competition, however, foliar [N] increased and δ15N decreased in response to N-fertilization. Grass biomass accumulation was also unchanged by fertilisation, despite increases in foliar [N] and decreases in δ15N.
Conclusions
The N2-fixation capacity of A. karroo provided no growth advantage over non-legumes. The lack of responsiveness of biomass accumulation by both the woody species and the grasses to N-fertilization, despite evidence that plants accessed the N-fertilizer, indicates limitation by other nutrients.






Similar content being viewed by others
References
Boddey RM, Peoples MB, Palmer B, Dart PJ (2000) Use of the 15N natural abundance technique to quantify biological nitrogen fixation by woody perennials. Nutr Cycl Agroecosyst 57:235–270
Bond WJ (2008) What limits trees in C4 grasslands and savannas? Annu Rev Ecol Evol Syst 39:641–659
Bransby DI, Tainton NM (1977) The disc-pasture meter: possible applications in grazing management. Proc Grassl Soc S Afr 12:115–118
Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Brown JR, Archer S (1999) Shrub invasion of grassland: recruitment in continuous and not regulated by herbaceous biomass or density. Ecology 80:2385–2396
Chapin FS III, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. BioScience 37:49–57
Craine JM (2009) Resource strategies of wild plants. Princeton University Press, Princeton
Cramer MD (2012) Unravelling the limits to tree height: a major role for water and nutrient trade-offs. Oecologia 169:61–72
Cramer MD, Chimphango SBM, van Cauter A, Waldram MS, Bond WJ (2007) Grass competition induces N2 fixation in some species of African Acacias. J Ecol 95:1123–1133
Cramer MD, Hoffmann V, Verboom GA (2008) Nutrient availability moderates transpiration in Ehrharta calycina. New Phytol 179:1048–1057
Cramer MD, Hawkins HJ, Verboom GA (2009) The importance of nutritional regulation of plant water flux. Oecologia 161:15–24
Cramer MD, van Cauter A, Bond WJ (2010) Growth of N2-fixing African savanna Acacia species is constrained by below-ground competition with grass. J Ecol 98:156–167
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Evol Syst 33:507–559
Ehleringer JR, Rundel PW (1989) History, units and instrumentation. In: Rundel PW, Ehleringer JR, Nagy KA (eds) Stable isotopes in ecological research. Ecol Stu 68. Springer, New York, pp 1–15
Evans DR (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6:121–126
Everard K, Seabloom EW, Harpole WS, de Mazancourt C (2010) Plant water use affects competition for nitrogen: why drought favors invasive species in California. Am Nat 175:85–97
Field C, Merino J, Mooney HA (1983) Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 60:384–389
Fredeen AL, Gamon JA, Field CB (1991) Responses of photosynthesis and carbohydrate-partitioning to limitation in nitrogen and water availability in field-grown sunflower. Plant Environ 14:963–970
Frost P, Medina E, Menaut J-C, Solbrig O, Swift M, Walker B (1986) Responses of savannas to stress and disturbance. Biol Int Spec Issue 10:1–82
Higgins SI, Bond WJ, Trollope WSW (2000) Fire, resprouting and variability, a recipe for grass–tree coexistence in savanna. J Ecol 88:213–229
Hoffmann MT, Ashwell A (2001) Nature divided: land degradation in South Africa. University of Cape Town Press, Cape Town
Högberg P (1997) 15N natural abundance in soil–plant systems. New Phytol 137:179–203
House JI, Archer S, Breshears DD, Scholes RJ, NCEAS Tree–Grass Interactions Participants (2003) Conundrums in mixed woody-herbaceous plant systems. J Biogeogr 30:1763–1777
Jurena PN, Archer S (2003) Woody plant establishment and spatial heterogeneity in grasslands. Ecology 84:907–919
Kahmen A, Wanek W, Buchmann N (2008) Foliar δ15N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia 156:861–870
Kalra YP (1998) Handbook of standard methods of plant analysis. CRC Press, Boca Raton
Kambatuku JR, Cramer MD, Ward D (2010) Intraspecific competition between shrubs in a semi-arid savanna. Plant Ecol 212:701–713
Knoop WT, Walker BH (1985) Interactions of woody and herbaceous vegetation in a Southern African savanna. J Ecol 73:235–253
Lata J-C, Degrange V, Raynaud X, Maron P-A, Lensi R, Abbadie L (2004) Grass populations control nitrification in savanna soils. Funct Ecol 18:605–611
Lewis GP (2005) Acacieae. In: Lewis G, Schrire B, Mackinder B, Lock M (eds) Legumes of the world. Royal Botanic Gardens, Kew, pp 187–192
Meyer KM, Ward D, Wiegand K, Moustakas A (2008) Multi-proxy evidence for competition between savanna woody species. Perspect Plant Ecol 10:63–72
Midgley JM, Bond WJ (2001) A synthesis of the demography of African acacias. J Trop Ecol 17:871–886
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. SANBI, Pretoria
O’Connor TG, Crow VRT (2000) Rate and pattern of bush encroachment in Eastern Cape savanna and grassland. Afr J Range For Sci 16:26–31
Patterson TB, Guy RD, Dang QL (1997) Whole-plant nitrogen- and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia 110:160–168
Raven JA, Handley LL, Wollenweber B (2004) Plant nutrition and water use efficiency. In: Bacon MS (ed) Water use efficiency in plant biology. Blackwell, Oxford, pp 162–188
Riginos C (2009) Grass competition suppresses savanna tree growth across multiple demographic stages. Ecology 90:335–340
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Sankaran M, Ratnam J, Hanan NP (2005) Tree–grass coexistence in savannas revisited—insights from an examination of assumptions and mechanisms invoked in existing models. Ecol Lett 7:480–490
Sankaran M, Ratnam J, Hanan N (2008) Woody cover in African savannas: the role of resources, fire and herbivory. Glob Ecol Biogeogr 17:236–245
Scholes RJ, Archer SR (1997) Tree–grass interactions in savannas. Annu Rev Ecol Evol Syst 28:517–544
Seibt U, Rajabi A, Griffiths H, Berry JA (2008) Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155:441–454
Sharam G, Sinclair ARE, Turkington R (2006) Establishment of broad-leaved thickets in Serengeti, Tanzania: the influence of fire, browsers, grass competition, and elephants. Biotropica 38:599–605
Tilman D (1987) Secondary succession and the pattern of plant dominance along Exp. nitrogen gradients. Ecol Monogr 57:189–214
Walker BH, Noy-Meir I (1982) Aspects of stability and resilience of savanna ecosystem. In: Walker BH, Huntley BJ (eds) Ecology of tropical savannas. Springer, Berlin, pp 556–590
Walter H (1971) Ecology of tropical and subtropical vegetation. Oliver and Boyd, Edinburg
Wang L, D’Odorico P, Ringrose S, Coetzee S, Macko SA (2007) Biogeochemistry of Kalahari sands. J Arid Environ 71:259–279
Wiegand K, Saltz D, Ward D (2006) A patch-dynamics approach to savanna dynamics and woody plant encroachment—insights from an arid savanna. Perspect Plant Ecol 7:229–242
Wigley BJ, Bond WJ, Hoffman MT (2010) Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob Change Biol 16:964–976
Acknowledgments
We thank Julia Wakeling, Linda Nell and the field staff of the Zululand Tree Project for field assistance and Ian Newton (Department Archeometry, University of Cape Town) for mass spectrometer analysis. Ezemvelo KZN Wildlife staff are thanked for assistance and permission to work in Hluhluwe-iMfolozi nature reserve. We are grateful for funding from the Mellon Foundation and National Research Foundation. We are also grateful to the reviewers for extensive and helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online resource 1
Initial and post-harvest soil characteristics (mean ± SE) averaged across all plots after determining that there were no significant differences between soils of various treatments. Soils were dried at 70 °C for 48 h prior to analysis. Different letters indicate significant differences (P < 0.05) as determined by two-way ANOVA. (DOCX 28 kb)
Online resource 2
Clipped (left) and unclipped (right) grass foliar a, b) δ13C values, c, d) δ15N values, e, f) [P] and g, h) [N] of foliar samples collected regularly throughout the growing period. Arrows indicate the addition of N fertilizer at three times during the growing period. Symbols represent the mean ± SE (n = 3 plots) (DOCX 446 kb)
Online resource 3
Contribution of fertilizer N to the N-budgets of both clipped (left) and unclipped (right) grass estimated on the basis of δ15N values of foliar samples collected regularly throughout the growing period. Arrows indicate the addition of N fertilizer at three times during the growing period. Symbols represent the mean ± SE (DOCX 65 kb)
Rights and permissions
About this article
Cite this article
Cramer, M.D., Bond, W.J. N-fertilization does not alleviate grass competition induced reduction of growth of African savanna species. Plant Soil 366, 563–574 (2013). https://doi.org/10.1007/s11104-012-1456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1456-4



