Abstract
Background and Aim
Root-derived carbon (C) is preferentially retained in soil compared to aboveground C inputs. Microbial communities in the rhizosphere are crucial to nutrient and organic matter cycling within agroecosystems. The overall aim of this study was to investigate the impacts of crop management on microbial community structure and processing of rhizodeposit-C within microenvironments of two soil zones, the rhizosphere versus non-rhizosphere.
Methods
New root-C (Cnew) from 13C-labeled hairy vetch (Vicia dasycarpa) plants were traced into phospholipid fatty acids (PLFA) within microaggregate (53–250 μm) and silt-and-clay (<53 μm) microenvironments in rhizosphere and non-rhizosphere soil during the cover crop growing season in long-term conventional (annual synthetic fertilizer applications), low-input (synthetic fertilizer and cover crop applied in alternating years), and organic (annual composted manure and cover crop additions) maize-tomato systems (Zea mays L.- Lycopersicum esculentum L.).
Results
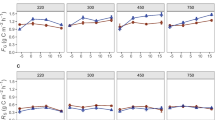
Among the three cropping systems, the composition of the microbial communities processing root-derived C were similar, which implied that the cropping systems maintained diverse microbial communities that were capable of utilizing similar C substrates despite receiving different long-term nutrient inputs. Relative distributions of root-derived PLFA-C (13C mol%) in the rhizosphere and non-rhizosphere were not significantly different, thereby suggesting that the structure of the microbial community utilizing new root-C in the rhizosphere- and non-rhizosphere microenvironments were similar. However, total PLFA biomass was four times greater, and root-derived PLFA-C in both soil microenvironments were approximately 10 times greater in the rhizosphere than in the non-rhizosphere. Although no microbial group dominated the processing of Cnew in the microenvironments of the rhizosphere and non-rhizosphere, we found that the microbial community of the silt-and-clay in the rhizosphere played a different role in the cycling of Cnew compared to communities in the rhizosphere microaggregates and those in the silt-and-clay and microaggregates of the non-rhizosphere.
Conclusions
Our results confirm that rhizodeposition plays an important role in the greater contribution of root-C than residue-C to SOM stabilization. This study also showed that microbial communities assimilating rhizodeposit-C are sensitive to their microenvironment (i.e., microaggregates versus silt-and-clay particles); nevertheless, differences in long-term crop management did not lead to differences in the capacity of the microbial communities to utilize active cover crop root-C substrates.





Similar content being viewed by others
References
Angers DA, Caron J (1998) Plant-induced changes in soil structure: processes and feedbacks. Biogeochem 45:55–72
Balesdent J, Balabane M (1996) Major contribution of roots to soil carbon storage inferred from maize cultivated soils. Soil Biol Biochem 28:1261–1263
Balser TC, Kinzig AP, Firestone MK (2002) Linking soil microbial communities and ecosystem functioning. In: Kinzig A, Pacala S, Tilman D (eds) The functional consequences of biodiversity. Princeton University Press, Princeton, pp 265–293
Bardgett RD, McAlister E (1999) The measurement of soil fungal:bacterial biomass ratios as an indicator of ecosystem self-regulation in temperate meadow grasslands. Biol Fertil Soils 29:282–290
Barea J-M, Pozo MJ, Azcón R, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778
Beare MH, Parmelee RW, Hendrix PF, Cheng W, Coleman DD, Crossley DA Jr (1992) Microbial and faunal interactions and effects on litter nitrogen and decomposition in agroecosystems. Ecol Monogr 62:569–591
Bossio DA, Scow KM (1998) Impact of carbon and flooding on soil microbial communities: phosholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Bossio DA, Scow KM, Gunapala N, Graham KJ (1998) Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microb Ecol 36:1–12
Bowen GD, Rovira AD (1976) Microbial colonization of plant roots. Annu Rev Phytopathology 14:121–144
Bowen GD, Rovira AD (1999) The rhizosphere and its management to improve plant growth. Adv Agron 66:1–102
Bronick C (2005) Soil structure and management: a review. Geoderma 124:3–22
Butler JL, Williams MA, Bottomley PJ, Myrold DD (2003) Microbial community dynamics associated with rhizosphere carbon flow. Appl Environ Microbiol 69:6793–6800
Buyer JS, Kaufman DD (1997) Microbial diversity in the rhizosphere of corn grown under conventional and low-input systems. Appl Soil Ecol 5:21–27
Denef K, Roobroeck D, Manimel Wadu MCW, Lootens P, Boeckx P (2009) Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol Biochem 41:144–153
Drinkwater LE, Snapp SS (2007) Nutrients in agroecosystems: rethinking the management paradigm. In: Donald LS (ed) Advances in agronomy. Academic Press, pp 163–186
Elfstrand S, Lagerlöf J, Hedlun K, Mårtensson A (2008) Carbon routes from decomposing plant residues and living roots into soil food webs assessed with 13C labeling. Soil Biol Biochem 40:2530–2539
Elliott ET (1986) Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci Soc Am J 50:627–633
Elliott ET, Anderson RV, Coleman DC, Cole CV (1980) Habitable pore space and microbial trophic interactions. Oikos 35:327–335
Frey SD, Elliott ET, Paustian K (1999) Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol Biochem 31:573–585
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Grayston SJ, Wang S, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378
Grayston SJ, Griffith GS, Mawdsley JL, Campbell CD, Bardgett RD (2001) Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol Biochem 33:533–551
Gunapala N, Scow KM (1998) Dynamics of soil microbial biomass and activity in conventional and organic farming systems. Soil Biol Biochem 30:805–816
Gupta VVSR, Germida JJ (1988) Distribution of microbial biomass and its activity in different soil aggregate size classes as affected by cultivation. Soil Biol Biochem 20:777–786
Hailer T, Stolp H (1985) Quantitative estimation of root exudation of maize plants. Plant Soil 86:207–216
Hirsch PR, Gilliam LM, Sohi SP, Williams JK, Clark IM, Murray PJ (2009) Starving the soil of plant inputs for 50 years reduces abundance by not diversity of soil bacterial communities. Soil Biol Biochem 41:2024–2121
Högberg MN, Chen Y, Högberg P (2007) Gross nitrogen mineralization and fungi-to-bacteria ratios are negatively correlated in boreal forests. Biol Fertil Soils 44:363–366
Kandeler E, Murer E (1993) Aggregate stability and soil microbial processes in a soil with different cultivation. Geoderma 56:503–513
Kandeler E, Kampichler C, Horak O (1996) Influence of heavy metals on the functional diversity of soil microbial. Biol Fertil Soils 23:299–306
Kennedy AC (1998) The rhizosphere and spermosphere. In: Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA (eds) Principles and applications of soil microbiology. Prentice Hall, Upper Saddle River, pp 389–407
Kent AD, Triplett EW (2002) Microbial communities and their interactions in soil and rhizosphere ecosystems. Annu Rev Microbiol 56:211–236
Kong AYY, Six J (2010) Tracing cover crop root versus residue carbon into soils from conventional, low-input, and organic cropping systems. Soil Sci Soc Am J 74:1201–1210
Kong AYY, Six J (2011) Protocol: isolation of biophysical microenvironments from rhizosphere and non-rhizosphere soil. Proetheuswiki http://prometheuswikipublishcsiroau/tikicustom_homephp
Kong AYY, Scow KM, Córdova-Kreylos AL, Holmes WE, Six J (2011) Microbial community composition and carbon cycling within soil microenvironments of conventional, low-input, and organic cropping systems. Soil Biol Biochem 43:20–30
Kuikman PJ, van Elsas JD, Jansen AG, Burgers SLGE, van Veen JA (1990) Population dynamics and activity of bacterial and protozoa in relation to their spatial distribution in soil. Soil Biol Biochem 22:1063–1073
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review. J Plant Nutr Soil Sci 163:421–431
Kuzyakov Y, Schneckenberger K (2004) Revier of estimation of plant rhizodeposition and their contribution to soil organic matter formation. Arch Acker Pfl Boden 50:115–132
Lu YH, Abraham W-R, Conrad R (2007) Spatial variation of active microbiota in the rice rhizosphere revealed by in situ stable isotope probing of phospholipid fatty acids. Environ Microbiol 9:474–481
Lundquist EJ, Scow KM, Jackson LE, Uesugi SL, Johnson CR (1999) Rapid response of soil microbial communities from conventional, low input and organic farming systems to a wet/dry cycle. Soil Biol Biochem 31:1661–1675
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10
Michulmas DG, Lauenroth WK, Singh JS, Cole CV (1985) Root turnover and production by 14C dilution: implications of carbon partitioning in plants. Plant Soil 88:353–365
Miller M, Dick RP (1995) Dynamics of soil C and microbial biomass in whole soil and aggregates in two cropping systems. Appl Soil Ecol 2:253–261
Miller RM, Jastrow JD (2000) Mycorrhizal fungi influence soil structure. In: Kapulnik Y, Douds DD Jr. (eds) Arbuscular mycorrhizas: physiology and function. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 3–18
Oades JM (1978) Mucilages at the root surface. J Soil Sci 29:1–16
Paterson E (2003) Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur J Soil Sci 54:741–750
Poly F, Ranjard L, Nazaret S, Gourbiere F, Jocteur-Monrozier L (2001) Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl Environ Microbiol 67:2255–2262
Postma J, van Veen JA, Watler S (1989) Influence of different initial soil moisture contents on the distribution and population dynamics of introduced Rhizobium leguminosarum biovar Trifolii. Soil Biol Biochem 21:437–442
Powlson DS, Prookes PC, Christensen BT (1987) Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol Biochem 19:159–164
Ranjard L, Richaume AS (2001) Quantitative and qualitative microscale distribution of bacteria in soil. Res Microbiol 152:707–716
Ranjard L, Poly F, Combrisson J, Richaume A, Gourbière F, Thioulouse J, Nazaret S (2000) Heterogeneous cell density and genetic structure of bacterial pools associated with various soil microenvironments as determined by enumeration and DNA fingerprinting approach (RISA). Microb Ecol 39:263–272
Rasse DP, Rumpel C, Dignac M-F (2005) Is soil carbon mostly root carbon? mechanisms for a specific stabilization. Plant Soil 269:341–356
SAS Institute (2002) Statistical analysis system. Version 92 SAS Institute, Cary
Saxton AM (1998) A macro for converting mean separation output to letter groupings in Proc Mixed. In: Proc 23rd SAS Users Group Intl, SAS Institute, Cary, North Carolina, pp 1243–1246
Schutter ME, Dick RP (2002) Microbial community profiles and activities among aggregates of winter fallow and cover-cropped soils. Soil Sci Soc Am J 66:142–153
Six J, Elliott ET, Paustian K (2000) Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem 32:2099–2103
Six J, Feller C, Denef K, Ogle SM, Sa MJC, Albrecht A (2002) Soil organic matter, biota and aggregation in temperate and tropical soils–effects of no-tillage. Agronom Agricult Environ 22:755–775
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569
Swinnen J, van Veen JA, Merckx R (1994) Rhizosphere carbon fluxes in field grown spring wheat: Model calculations based on 14C partitioning after pulse-labeling. Soil Biol Biochem 26:171–182
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163
Treonis AM, Ostle NJ, Stott AW, Primrose R, Grayston SJ, Ineson P (2004) Identification of groups of metabolically-active rhizosphere microorganisms by stable isotope probing of PLFAs. Soil Biol Biochem 36:533–537
van Ginkel JH, Gorissen A, Polci D (2000) Elevated atmospheric carbon dioxide concentration: effects of increased carbon input in a Lolium perenne soil on microorganisms and decomposition. Soil Biol Biochem 32:449–456
Vargas R, Hattori T (1986) Protozoan predation of bacterial cells in soil aggregates. FEMS Microbiol Ecol 38:233–242
Warembourg FR, Paul EA (1973) The use of 14CO2 canopy techniques for measuring carbon transfers through the plant-root-soil system. Plant Soil 38:33l–345l
Zelles L (1997) Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275–294
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in characterization of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Acknowledgements
We thank Engil Isadora Pujol Pereira, Alice Yan, and Teresa Yim for their assistance in the laboratory; Dennis Bryant, Israel Herrera, and the field crew at the Russell Ranch for their help in the field; and the PLFA staff in the lab of Dr. Kate Scow for their cooperation. This research was supported by a grant from the Kearney Foundation of Soil Science, University of California, and a graduate student fellowship from the Western Sustainable Agriculture Research and Education Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson.
Rights and permissions
About this article
Cite this article
Kong, A.Y.Y., Six, J. Microbial community assimilation of cover crop rhizodeposition within soil microenvironments in alternative and conventional cropping systems. Plant Soil 356, 315–330 (2012). https://doi.org/10.1007/s11104-011-1120-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1120-4