Abstract
Background and aims
Rice (Oryza sativa L.) is the primary source of carbohydrate for the majority of the World's population. Herbaspirillum seropedicae is a diazotroph that lives within and on the surface of rice roots. It can promote the growth of rice, partly by supplying it with fixed nitrogen.
Methods
To better understand the rice–H. seropedicae interaction, cDNA libraries from rice roots either inoculated (RRCH) or uninoculated (RRSH) with the diazotroph were obtained and analysed.
Results
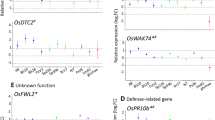
Potential differentially expressed genes identified from the libraries encoded a metallothionein-like protein type 1, a NOD26-like membrane integral protein ZmNIP2-1, a thionin family protein, an oryzain gamma chain precursor, stress-associated protein 1 (OsISAP1), probenazole-inducible protein PBZ1 and auxin- and ethylene-responsive genes. Differential expression was analysed by qRT-PCR for some of these genes and confirmed in most cases. The expression of stress- and defence-related genes coding for thionins, PBZ1 and OsISAP1 was repressed, while expression of a metallothionein gene was induced by inoculation with H. seropedicae. In contrast, expression of auxin-responsive genes was repressed, while expression of ethylene genes was either repressed or induced. The possible involvement of these and other genes in plant-bacterial interactions is discussed.
Conclusions
The decrease in expression of the defence-related proteins PBZ1 and thionins in the rice–H. seropedicae association, suggests that the bacteria modulate plant defence responses during colonisation. The expression of genes responsive to auxin and ethylene also appears to be regulated by the bacteria.



Similar content being viewed by others
Abbreviations
- EST:
-
Expressed sequence tag
- PGPR:
-
Plant-growth promoting rhizobacteria
- PRR:
-
Pattern recognition receptor
- PAMP:
-
Pathogen-associated molecular pattern
- RAP-DB:
-
Rice annotation project-database
- GO:
-
Gene ontology
- NBS:
-
Nucleotide-binding site
- ERF:
-
Ethylene response factor
- ARF:
-
Auxin response factor
- PBZ1:
-
Probenazole-inducible gene
- OsISAP1:
-
Ozyza sativa subspecies indica stress-associated protein
- EAS:
-
5-Epi-aristolochene synthase
- RLK:
-
Receptor-like protein kinase
- T3SS:
-
Type three secretion system
- IAA:
-
Indole acetic acid
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arencibia AD, Vinagre F, Estevez Y, Bernal A, Perez J, Cavalcanti J, Santana I, Hemerly AS (2006) Gluconacetobacter diazotrophicus elicits a sugarcane defense response against a pathogenic bacteria Xanthomonas albilineans. Plant Signal Behav 1:265–273
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25:25–29
Baldani JJ, Baldani VLD, Seldin L, Dobereiner J (1986) Characterization of Herbaspirillum seropedicae gen. nov., sp. nov., a root-associated nitrogen-fixing bacterium. Int J Syst Bacteriol 36:86–93
Bastian F, Cohen A, Piccoli P, Luna V, Baraldi R, Bottini R (1998) Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically-defined culture media. Plant Growth Regul 24:7–11
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL (2008) GenBank. Nucleic Acids Res 36:D25–D30
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Bohlmann H, Clausen S, Behnke S, Giese H, Hiller C, Reimann-Philipp U, Schrader G, Barkholt V, Apel K (1988) Leaf-thionins of barley: a novel class of cell wall proteins toxic to plant-pathogenic fungi and possibly involved in the defense mechanism of plants. EMBO J 7:1559–1565
Cavalcante JJV, Vargas C, Nogueira EM, Vinagre F, Schwarcz K, Baldani JI, Ferreira PCG, Hemerly AS (2007) Members of the ethylene signaling pathway are regulated in sugarcane during the association with nitrogen-fixing endophytic bacteria. J Exp Bot 58:673–686
Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol 129:661–677
Ciardi JA, Tieman DM, Lund ST, Jones JB, Stall RE, Klee HJ (2000) Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol 123:81–92
Deakin WJ, Broughton WJ (2009) Symbiotic use of pathogenic strategies: rhizobial protein secretion systems. Nat Rev Microbiol 7:312–320
DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7:1243–1249
Ewing B, Green P (1998) Base-calling of automated sequencer traces using Phred. II. Error Probabilities. Genome Res 8:186–194
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using Phred. I. Accuracy Assessment. Genome Res 8:175–185
Florack DEA, Stiekema WJ (1994) Thionins: properties, possible biological roles and mechanisms of action. Plant Mol Biol 26:25–37
Gómez-Gómez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5:1003–1011
Gutterson N, Reuber TL (2004) Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 7:465–471
Gyaneshwar P, James EK, Reddy PM, Ladha JK (2002) Herbaspirillum colonization increases growth and nitrogen accumulation in aluminium tolerant rice varieties. New Phytol 154:131–146
Hannah MA, Heyer AG, Hincha DK (2005) A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLOS Genet 1:179–196
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agricultural Experiment Station, California, circular 347
Hoffman T, Schmidt JS, Zheng X, Bent AF (1999) Isolation of ethylene-insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene disease resistance. Plant Physiol 119:935–949
Hofius D, Maier AT, Dietrich C, Jungkunz I, Bornke F, Maiss E, Sonnewald U (2007) Capsid protein-mediated recruitment of host DnaJ-like proteins is required for Potato virus Y infection in tobacco plants. J Virol 81:11870–11880
Hong SW, Jon JH, Kwak JM, Nam HG (1997) Identification of a receptor-like protein kinase gene rapidly induced by abscisic acid, dehydration, high salt and cold treatments in Arabidopsis thaliana. Plant Physiol 113:1203–1212
Huang X, Madan A (1999) CAP3: A DNA sequence assembly program. Genome Res 9:868–877
Itoh T, Tanaka T, Barrero RA et al (2007) Curated genome annotation of Oryza sativa ssp. japonica and comparative genome analysis with Arabidopsis thaliana. Genome Res 17:175–183
Jain M, Khurana JP (2009) Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J 276:3148–3162
Jain M, Kaur N, Garg R, Thakur JK, Tyagi AK, Khurana JP (2006a) Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa). Funct Integr Genomics 6:47–59
Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006b) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345:646–651
James EK, Gyaneshwar P, Mathan N, Barraquio W, Reddy PM, Iannetta PPM, Olivares FL, Ladha JK (2002) Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant Microbe Interact 15:894–906
Jin S, Cheng Y, Guan Q, Liu D, Takano T, Liu S (2006) A metallothionein-like protein of rice (rgMT) functions in E. coli and its gene expression is induced by abiotic stresses. Biotechnol Lett 28:1749–1753
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kim S, Ahn IPL, Lee YH (2001) Analysis of genes expressed during rice–Magnaporthe grisea interactions. Mol Plant Microbe Interact 14:1340–1346
Kim ST, Kim SG, Kang YH, Wang Y, Kim JY, Yi N, Kim JK, Rakwal R, Koh HJ, Kang KY (2008) Proteomics analysis of rice lesion mimic mutant (spl1) reveals tightly localized probenazole-induced protein (PBZ1) in cells undergoing programmed cell death. J Proteome Res 7:1750–1760
Ladha JK, Reddy PM (2003) Nitrogen fixation in rice systems: state of knowledge and future prospects. Plant Soil 252:151–167
Linder P, Owttrim GW (2009) Plant RNA helicases: linking aberrant and silencing RNA. Trends Plant Sci 14:344–352
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods 25:402–408
Lu L, Du Z, Qin M, Wang P, Lan H, Niu X, Jia D, Xie L, Lin Q, Xie L, Wu Z (2009) Pc4, a putative movement protein of Rice stripe virus, interacts with a type I DnaJ protein and a small Hsp of rice. Virus Genes 38:320–327
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Malarvizhi P, Ladha JK (1999) Influence of available nitrogen and rice genotype on associative nitrogen fixation. Soil Sci Soc Am J 63:93–99
Mukhopadhyay A, Vij S, Tyagi AK (2004) Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc Natl Acad Sci USA 101:6309–6314
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Olivares FL, Baldani VLD, Reis VM, Baldani JI, Döbereiner J (1996) Occurrence of the endophytic diazotrophs Herbaspirillum spp. in roots, stems and leaves predominantly of Gramineae. Biol Fertil Soils 21:197–200
Pedrosa FO, Monteiro RA, Wassem R, Cruz LM, Ayub RA et al (2011) Genome of Herbaspirillum seropedicae strain SmR1, a specialized diazotrophic endophyte of tropical grasses. PLoS Genet 7:e1002064
Pereira JAR, Cavalcante VA, Baldani JI, Dobereiner J (1988) Field inoculation of sorghum and rice with Azospirillum spp. and Herbaspirillum seropedicae. Plant Soil 110:269–274
Rogg LE, Bartel B (2001) Auxin signaling: derepression through regulated proteolysis. Dev Cell 1:595–604
Roncato-Maccari LDB, Ramos HJO, Pedrosa FO, Alquini Y, Chubatsu LS, Yates MG, Rigo LU, Steffens MBR, Souza EM (2003) Endophytic Herbaspirillum seropedicae expresses nif genes in gramineous plants. FEMS Microbiol Ecol 45:39–47
Stec B (2006) Plant thionins - the structural perspective. Cell Mol Life Sci 63:1370–1385
Takemoto D, Hayashi M, Noriyuki D, Nishimura M, Kawakita K (1999) Molecular cloning of a defense-response cytochrome P450 gene from tobacco. Plant Cell Physiol 40:1232–1242
Verhage A, van Wees SC, Pieterse CM (2010) Plant immunity: it's the hormones talking, but what do they say? Plant Physiol 154:536–540
Vinagre F, Vargas C, Schwarcz K, Cavalcante J, Nogueira EM, Baldani JI, Ferreira PCG, Hemerly AS (2006) SHR5: a novel plant receptor kinase involved in plant-N2-fixing endophytic bacteria association. J Exp Bot 57:559–569
Wang D, Pei K, Fu Y, Sun Z, Li S, Liu H, Tang K, Han B, Tao Y (2007) Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 394:13–24
Wang D, Pan Y, Zhao X, Zhu L, Fu B, Li Z (2011) Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice. BMC Genomics 12:149–164
Weber OB, Baldani VLD, Teixeira KRS, Kirchhof G, Baldani JI, Dobereiner J (1999) Isolation and characterization of diazotrophic bacteria from banana and pineapple plants. Plant Soil 210:103–113
Wu J, Maehara T, Shimokawa T et al (2002) A comprehensive rice transcript map containing 6591 expressed sequence tag sites. Plant Cell 14:525–535
Zhong L, Wang K, Tan J, Li W, Li S (2002) Putative cytochrome P450 genes in rice genome (Oryza sativa L. ssp. indica) and their EST evidence. Sci China Ser C 45:512–517
Acknowledgments
We are grateful to Roseli Prado and Julieta Pie for technical support, Embrapa Arroz Feijão and Instituto Riograndense do Arroz for providing seeds. We also thank Euan K. James for revising the final version of this manuscript. This work was supported by CNPq-Instituto do Milênio, INCT-FBN, CNPq and CAPES.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Euan K. James.
Rights and permissions
About this article
Cite this article
Brusamarello-Santos, L.C.C., Pacheco, F., Aljanabi, S.M.M. et al. Differential gene expression of rice roots inoculated with the diazotroph Herbaspirillum seropedicae . Plant Soil 356, 113–125 (2012). https://doi.org/10.1007/s11104-011-1044-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1044-z