Abstract
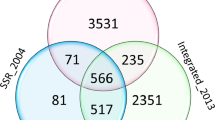
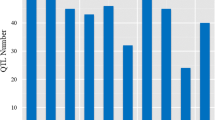
Traits related to the root length of maize (Zea mays L.), reported by 15 QTL studies of nine mapping populations, were subjected to a QTL meta-analysis. Traits were grouped according to ontology, and we propose a system of abbreviations to unambiguously identify the different root types and branching orders. The nine maps were merged into a consensus map, and the number and positions of putative QTL clusters (MQTLs) were determined. A total of 161 QTLs was grouped into 24 MQTLs and 16 individual QTLs. Seven MQTLs harbored root traits, which had been reported to be collocated with QTLs for grain yield or other drought-responsive traits in the field. The most consistent collocations were observed for the number and weight of the seminal roots (five loci). Based on our analysis at least six loci are good candidates for further evaluation (bins 1.07, 2.04, 2.08, 3.06, 6.05 and 7.04). For example, the MQTL in bin 2.04 harbored ten different single QTLs; the MQTLs in bins 1.07 and 3.06 combined 11 and 7 QTLs, respectively, that were detected in more than three populations. The presented database is a first step for a comprehensive overview of the genetic architecture of root system architecture and its ecophysiological function.



Similar content being viewed by others
References
Araki H, Hirayama M, Hirasawa H, Iijima M (2000) Which roots penetrate the deepest in rice and maize root systems? Plant Prod Sci 3:281–288
Barrière Y, Gibelin C, Argillier O, Mechin V (2001) Genetic analysis in recombinant inbred lines of early dent forage maize. I—QTL mapping for yield, earliness, starch and crude protein contents from per se value and top cross experiments. Maydica 46:253–266
Bruce WB, Edmeades GO, Baker TC (2002) Molecular and physiological approaches to maize improvement for drought tolerance. J Exp Bot 53:13–25
Courtois B, Ahmadi N, Khowaja F, Price A, Rami J-F, Frouin J, Hamelin C, Ruiz M (2009) Rice root genetic architecture: meta-analysis from a drought QTL database. Rice 2:115–128
Donohue K (2009) Completing the cycle: maternal effects as the missing link in plant life histories. Philos. Trans. R. Soc. B-Biol. Sci. 364:1059–1074
de Dorlodot S, Forster B, Pages L, Price A, Tuberosa R, Draye X (2007) Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci 12:474–481
Duvick DN (2005) The contribution of breeding to yield advances in maize (Zea mays L.). In Adv Agron. Academic Press, pp 83–145
Enns LC, McCully ME, Canny MJ (2006) Branch roots of young maize seedlings, their production, growth, and phloem supply from the primary root. Funct Plant Biol 33:391–399
Feix G, Hochholdinger F, Park WJ (2000) Maize root system and genetic analysis of its formation. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker, New York, pp 205–220
Feldman L (1994) The maize root. In: Freeling M, Walbot V (eds) The maize handbook. Springer-Verlag, New York, pp 29–37
Fracheboud Y, Ribaut J-M, Vargas M, Messmer R, Stamp P (2002) Identification of quantitative trait loci for cold-tolerance of photosynthesis in maize (Zea mays L.). J Exp Bot 53:1967–1977
Gardiner JM, Coe EH, Meliahancock S, Hoisington DA, Chao S (1993) Development of a core RFLP map in maize using an immortalized-F2 population. Genetics 134:917–930
Girardin P, Jordan MO, Picard D, Trendel R (1986) Harmonization of terms used for the morphological description of maize. Agronomie 6:873–875
Giuliani S, Sanguineti MC, Tuberosa R, Bellotti M, Salvi S, Landi P (2005) Root-ABA1, a major constitutive QTL, affects maize root architecture and leaf ABA concentration at different water regimes. J Exp Bot 56:3061–3070
Giuliani S, Busatto C, Salvi S, Ricciolini C, Carraro N, Presterl T, Ouzunova M, Conti S, Sanguineti MC and Tuberosa R (2009) Genetic dissection of root characteristics at the seminal and seedling level in maize. In XXI International Conference of the EUCARPIA Maize and Sorghum Section, June 21–24, Bergamo, Italy
Goffinet B, Gerber S (2000) Quantitative trait loci: a meta-analysis. Genetics 155:463–473
Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, Young IM (2009) Root phenomics of crops: opportunities and challenges. Funct Plant Biol 36:922–929
Guingo A, Hébert Y, Charcosset A (1998) Genetic analysis of root traits in maize. Agronomie 18:225–235
Hargreaves CE, Gregory PJ, Bengough AG (2009) Measuring root traits in barley (Hordeum vulgare ssp vulgare and ssp spontaneum) seedlings using gel chambers, soil sacs and X-ray microtomography. Plant Soil 316:285–297
Hetz W, Hochholdinger F, Schwall M, Feix G (1996) Isolation and characterisation of rtcs, a mutant deficient in the formation of nodal roots. Plant J 10:845–857
Hochholdinger F (2008) The maize root system: morphology, anatomy, and genetics. In: Hake SC, Bennetzen JL (eds) Handbook of maize: its biology. Springer, The Netherlands, pp 145–160
Hochholdinger F, Feix G (1998) Early post-embryonic root formation is specifically affected in the maize mutant lrtl. Plant J 16:247–255
Hochholdinger F, Tuberosa R (2009) Genetic and genomic dissection of maize root development and architecture. Curr Opin Plant Biol 12:172–177
Hoecker N, Keller B, Piepho HP, Hochholdinger F (2006) Manifestation of heterosis during early maize (Zea mays L.) root development. Theor Appl Genet 112:421–429
Hoppe DC, McCully ME, Wenzel CL (1986) The nodal roots of Zea—their development in relation to structural features of the stem. Can J Bot-Revue Canadienne De Botanique 64:2524–2537
Hoshikawa K (1969) Underground organs of seedlings and systematics of Gramineae Botanical Gazette 130:192–203
Hund A (2010) Genetic variation in the gravitropic response of maize roots to low temperatures. Plant Root 4:22
Hund A, Frachboud Y, Soldati A, Frascaroli E, Salvi S, Stamp P (2004) QTL controlling root and shoot traits of maize seedlings under cold stress. Theor Appl Genet 109:618–629
Hund A, Richner W, Soldati A, Fracheboud Y, Stamp P (2007) Root morphology and photosynthetic performance of maize inbred lines at low temperature. Eur J Agron 27:52–61
Hund A, Ruta N, Liedgens M (2009a) Rooting depth and water use efficiency of tropical maize inbred lines, differing in drought tolerance. Plant Soil 318:311–325
Hund A, Trachsel S, Stamp P (2009b) Growth of axile and lateral roots of maize: I development of a phenotyping platform. Plant Soil 325:335–349
Ilic K, Kellogg EA, Jaiswal P, Zapata F, Stevens PF, Vincent LP, Avraham S, Reiser L, Pujar A, Sachs MM, Whitman NT, McCouch SR, Schaeffer ML, Ware DH, Stein LD, Rhee SY (2007) The plant structure ontology, a unified vocabulary of anatomy and morphology of a flowering plant. Plant Physiol 143:587–599
Jenkins MT (1930) Heritable characters of maize XXXIV—rootless. J Hered 21:79–80
Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Science 40:358–364
Kisselbach TA (1999) The structure and reproduction of corn, 50th anniversary ed. Cold Spring Harbor Labporatory Press, New York, USA
Landi P, Sanguineti MC, Darrah LL, Giuliani MM, Salvi S, Conti S, Tuberosa R (2002) Detection of QTLs for vertical root pulling resistance in maize and overlap with QTLs for root traits in hydroponics and for grain yield under different water regimes. Maydica 47:233–243
Landi P, Sanguineti MC, Liu C, Li Y, Wang TY, Giuliani S, Bellotti M, Salvi S, Tuberosa R (2007) Root-ABA1 QTL affects root lodging, grain yield, and other agronomic traits in maize grown under well-watered and water-stressed conditions. J Exp Bot 58:319–326
Landi P, Bellotti M, Ferri M, Giuliani S, Salvi S, Sanguineti MC, Tuberosa R (2009) Validation of a QTL on Bin 1.06 for root and gronomic traits in maize. In XXI International Conference of the EUCARPIA Maize and Sorghum Section, June 21–24, Bergamo, Italy
Landi P, Giuliani S, Salvi S, Ferri M, Tuberosa R, Sanguineti MC (2010) Characterization of root-yield-1.06, a major constitutive QTL for root and agronomic traits in maize across water regimes. J Exp Bot doi:10.1093/jxb/erq192
Lebreton C, Lazic Jancic V, Steed A, Pekic S, Quarrie SA (1995) Identification of QTL for drought responses in maize and their use in testing causal relationships between traits. J Exp Bot 46:853–865
Liu JC, Li JS, Chen FJ, Zhang FS, Ren TH, Zhuang ZJ and Mi GH (2008) Mapping QTLs for root traits under different nitrate levels at the seedling stage in maize (Zea mays L.). Plant and Soil 305:253–265
Loffler M, Schon CC, Miedaner T (2009) Revealing the genetic architecture of FHB resistance in hexaploid wheat (Triticum aestivum L.) by QTL meta-analysis. Mol Breed 23:473–488
Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55:493–512
Ma XQ, Tang JH, Teng WT, Yan JB, Meng YJ, Li JS (2007) Epistatic interaction is an important genetic basis of grain yield and its components in maize. Mol Breed 20:41–51
Mano Y, Omori F, Takamizo T, Kindiger B, Bird RM, Loaisiga CH, Takahashi H (2007) QTL mapping of root aerenchyma formation in seedlings of a maize x rare teosinte “Zea nicaraguensis” cross. Plant Soil 295:103–113
Manschadi AM, Hammer GL, Christopher JT, deVoil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129
Marandel G, Salava J, Abbott A, Candresse T, Decroocq V (2009) Quantitative trait loci meta-analysis of Plum pox virus resistance in apricot (Prunus armeniaca L.): new insights on the organization and the identification of genomic resistance factors. Mol Plant Pathol 10:347–360
McCully ME (1999) Roots in soil: unearthing the complexities of roots and their rhizospheres. Annu Rev Plant Physiol Plant Mol Biol 50:695–718
Messmer R (2006) The genetic dissection of key factors involved in the drought tolerance of tropical maize (Zea mays L.). Diss. ETH No. 16695., Zurich, Switzerland
Messmer R, Fracheboud Y, Banziger M, Vargas M, Stamp P, Ribaut JM (2009) Drought stress and tropical maize: QTL-by-environment interactions and stability of QTLs across environments for yield components and secondary traits. Theor Appl Genet 119:913–930
Nagel KA, Kastenholz B, Jahnke S, Van Dusschoten D, Aach T, Muhlich M, Truhn D, Scharr H, Terjung S, Walter A, Schurr U (2009) Temperature responses of roots: impact on growth, root system architecture and implications for phenotyping. Funct Plant Biol 36:947–959
Navara J, Jesko T, Duchoslav S (1994) Participation of seminal roots in water-uptake by maize root-system. Biologia 49:91–95
Nielsen KL, Lynch JK, Jablokow AG, Curtis PS (1994) Carbon cost of root systems: an architectural approach. Plant Soil 165:161–169
Omori F, Mano Y (2007) QTL mapping of root angle in F2 populations from maize ‘B73’ x teosinte ‘Zea luxurians’. Plant Root 1:57–64
O’Toole JC, Bland WL (1987) Genotypic variation in crop plant root systems. Adv Agron 41:91–145
Pages L, Jordan MO, Picard D (1989) A simulation-model of the 3-dimensional architecture of the maize root-system. Plant Soil 119:147–154
Passioura J (1972) Effect of root geometry on yield of wheat growing on stored water. Aust J Agric Res 23:745–752
Reimer R (2010) Fesponses of maize (Zea mays L.) seedlings to low and high temperature: association mapping of root growth and photosynthesis-related traits. In AGRL. Diss. ETH No. 18807, Zurich
Ribaut JM, Hoisington DA, Deutsch JA, Jiang C, Gonzalez de Leon D (1996) Identification of quantitative trait loci under drought conditions in tropical maize. 1. Flowering parameters and the anthesis-silking interval. Theor Appl Genet 92:905–914
Richards RA, Passioura JB (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain-yield in rain-fed environments. Aust J Agric Res 40:943–950
Ruta N, Liedgens M, Fracheboud Y, Stamp P, Hund A (2009) QTLs for the elongation of axile and lateral roots of maize in response to low water potential. Theor Appl Genet 120:621–631
Ruta N, Stamp P, Liedgens M, Fracheboud Y, Hund A (2010) Collocation of QTLs for seedling traits and yield components of tropical maize under limited water availability. Crop Sci 50:1385–1392
Sanguineti MC, Duvick DN, Smith S, Landi P, Tuberosa R (2006) Effects of long-term selection on seedling traits and ABA accumulation in commercial maize hybrids. Maydica 51:329–338
Sanguineti MC, Giuliani MM, Govi G, Tuberosa R and Landi P (1998) Root and shoot traits of maize inbred lines grown in the field and in hydroponic culture and their relationship with root lodging. Maydica 43:211–216
Sass JE (1977) Morphology. In: Sprague CF (ed) Corn and corn improvement. American Society of Agronomy, Madison, pp 89–110
Schaeffer ML, Sanchez-Villeda H, Coe E (2008) Neighbors 2008. In: Maize GDB http://www.maizegdb.org/cgi-bin/displayrefrecord.cgi?id=1204261 (27. Oct 2009)
Senior ML, Chin ECL, Lee M, Smith JSC, Stuber CW (1996) Simple sequence repeat markers developed from maize sequences found in the GENBANK database: map construction. Crop Sci 36:1676–1683
Shen L, Courtois B, McNally KL, Robin S, Li Z (2001) Evaluation of near-isogenic lines of rice introgressed with QTLs for root depth through marker-aided selection. Theor Appl Genet 103:75–83
Singh V, van Oosterom EJ, Jordan DR, Messina CD, Cooper M, Hammer GL (2010) Morphological and architectural development of root systems in sorghum and maize. Plant Soil 333:287–299
Steele KA, Price AH, Shashidhar HE, Witcombe JR (2006) Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor Appl Genet 112:208–221
Taramino G, Sauer M, Stauffer JL, Multani D, Niu XM, Sakai H, Hochholdinger F (2007) The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J 50:649–659
Trachsel S, Messmer R, Stamp P, Hund A (2009) Mapping of QTLs for lateral and axile root growth of tropical maize. Theor Appl Genet 119:1413–1424
Trachsel S, Kaeppler SM, Brown KM, Lynch J (2010) Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Submitted to Plant and Soil. Plant Soil in press
Tuberosa R, Sanguineti MC, Landi P, Salvi S, Casarini E, Conti S (1998) RFLP mapping of quantitative trait loci controlling abscisic acid concentration in leaves of drought-stressed maize (Zea mays L.). Theor Appl Genet 97:744–755
Tuberosa R, Salvi S, Sanguineti MC, Landi P, Maccaferri M, Conti S (2002a) Mapping QTLs regulating morpho-physiological traits and yield: case studies, shortcomings and perspectives in drought-stressed maize. Ann Bot 89:941–963
Tuberosa R, Sanguineti MC, Landi P, Giuliani MM, Salvi S, Conti S (2002b) Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Mol Biol 48:697–712
Tuberosa R, Salvi S, Sanguineti MC, Maccaferri M, Giuliani S, Maccaferri M, Sanguineti MC, Landi P, Tuberosa R, Giuliani S, Salvi S (2003) Searching for quantitative trait loci controlling root traits in maize: a critical appraisal. Plant Soil 255:35–54
Van Beem J, Smith ME, Zobel RW (1998) Estimating root mass in maize using a portable capacitance meter. Agron J 90:566–570
Veyrieras JB, Goffinet B, Charcosset A (2005) MetaQTL. In Version 1.0, France
Veyrieras JB, Goffinet B, Charcosset A (2007) MetaQTL: a package of new computational methods for the meta-analysis of QTL mapping experiments. BMC Bioinformatics 8
Watt M, Magee LJ, McCully ME (2008) Types, structure and potential for axial water flow in the deepest roots of field-grown cereals. New Phytol 178:135–146
Wojciechowski T, Gooding MJ, Ramsay L, Gregory PJ (2009) The effects of dwarfing genes on seedling root growth of wheat. J Exp Bot 60:2565–2573
Yazdanbakhsh N, Fisahn J (2009) High throughput phenotyping of root growth dynamics, lateral root formation, root architecture and root hair development enabled by PlaRoM. Funct Plant Biol 36:938–946
Zhu JM, Kaeppler SM, Lynch JP (2005) Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor Appl Genet 111:688–695
Zhu JM, Mickelson SM, Kaeppler SM and Lynch JP (2006) Detection of quantitative trait loci for seminal root traits in maize (Zea mays L.) seedlings grown under differential phosphorus levels. Theoretical and Applied Genetics 113:1–10
Zobel RW (2009) Report of the Root Nomenclature Workgroup ISRR V (1996) a Reprise. In Proceedings of the Seventh Symposium of the International Society for Root Research, September 4, 2009, Vienna. http://rootrap.boku.ac.at/index.php?id=28.
Zobel RW, Weisel Y (2010) A plant root system architectural taxonomy: a framework for root nomenclature. Plant Biosyst, in press
Acknowledgements
We would like to thank Prof. Dr. Peter Stamp for supporting this project and Marcia Schoenberg for linguistic corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter J. Gregory.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement 1
Meta QTLs (MQTLs) projected on the consensus map of chromosome 1 using MQTLView of MetaQTL. The most likely number of MQTLs per chromosome was estimated by QTLClust. The ID to link a QTL unambiguously to the original publication is given as [Experiment | Trait | LOD score | Chromosome position in cM ± Sign of the additivity]. For an example of this ID see Material and Methods (PNG 327 kb)
Supplement 2
MQTLs projected on the consensus map of chromosome 2. For details see Supplement 1. (PNG 273 kb)
Supplement 3
MQTLs projected on the consensus map of chromosome 3. For details see Supplement 1. (PNG 206 kb)
Supplement 4
MQTLs projected on the consensus map of chromosome 4. For details see Supplement 1. (PNG 221 kb)
Supplement 5
MQTLs projected on the consensus map of chromosome 5. For details see Supplement 1. (PNG 173 kb)
Supplement 6
MQTLs projected on the consensus map of chromosome 6. For details see Supplement 1. (PNG 157 kb)
Supplement 7
MQTLs projected on the consensus map of chromosome 7. For details see Supplement 1. (PNG 189 kb)
Supplement 8
MQTLs projected on the consensus map of chromosome 8. For details see Supplement 1. (PNG 155 kb)
Supplement 9
MQTLs projected on the consensus map of chromosome 9. For details see Supplement 1. (PNG 550 kb)
Supplement 10
MQTLs projected on the consensus map of chromosome 10. For details see Supplement 1. (PNG 249 kb)
Rights and permissions
About this article
Cite this article
Hund, A., Reimer, R. & Messmer, R. A consensus map of QTLs controlling the root length of maize. Plant Soil 344, 143–158 (2011). https://doi.org/10.1007/s11104-011-0735-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0735-9