Abstract
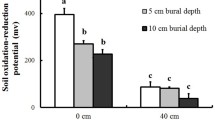
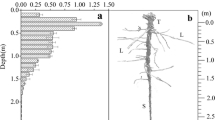
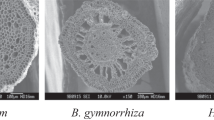
To understand the economics of root aerenchyma formation in wetland plants, we investigated in detail the response of Alisma triviale to waterlogging. We hypothesized costs being associated with development of a large root air space. In three out-door pot experiments, seedlings (1 experiment) and mature plants (2 experiments) were grown under waterlogged and drained conditions for up to 2 months. Waterlogging promoted growth, and was associated with increased root porosity and decreased root density (fresh mass per volume). The increased formation of aerenchyma was associated with a higher root dry matter content for a given root density. Despite improved growth and earlier flowering, the waterlogged plants also showed signs of being constrained by the anoxic substrate, such as shallower roots, and a higher leaf dry matter content. The formation of aerenchyma was associated with costs, such as increased root dry matter content and reduced metaxylem vessel diameter. The faster growth of the seedlings under the waterlogged conditions, despite some signs of being stressed, was possibly a result of decreased requirements to allocate biomass below ground. In mature plants the increased aerenchyma allowed deeper root penetration, and ameliorated the effects of anoxia, reducing the differences in plant traits between the treatments.




Similar content being viewed by others
References
Albrecht G, Mustroph A (2003) Localization of sucrose synthase in wheat roots: increased in situ activity of sucrose synthase correlates with cell wall thickening by cellulose deposition under hypoxia. Planta 217:252–260
Armstrong W (1979) Aeration in higher plants. Adv Bot Res 7:225–332
Armstrong W, Beckett PM (1987) Internal aeration and the development of stelar anoxia in submerged roots. A multishelled mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytol 105:221–245
Blom CWPM, Voesenek LACJ, Banga M, Engelaar WMHG, Rijnders JHGM, Van de Steeg HM, Visser EJW (1994) Physiological ecology of riverside species: adaptive responses of plants to submergence. Ann Bot 74:253–263
Cahn MD, Zobel RW, Bouldin DR (1989) Relationship between root elongation rate and diameter and duration of growth of lateral roots of maize. Plant Soil 119:271–279
Carter MF, Grace JB (1990) Relationships between flooding tolerance, life history, and short-term competitive performance in three species of Polygonum. Am J Bot 77:381–387
Carter EB, Theodorou MK, Morris P (1997) Responses of Lotus corniculatus to environmental change. I. Effects of elevated CO2, temperature and drought on growth and plant development. New Phytol 136:245–253
Chen H, Robert G, Qualls RG, Miller GC (2002) Adaptive responses of Lepidium latifolium to soil flooding: biomass allocation, adventitious rooting, aerenchyma formation and ethylene production. Environ Exp Bot 48:119–128
Colmer TD (2003) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deepwater rice (Oryza sativa L.). Ann Bot 91:301–309
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48:223–250
Ehlert C, Maurel C, Tardieu F, Simonneau T (2009) Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol 150:1093–1104
Engelaar WMHG, Van Bruggen MW, Van den Hoek WPM, Huyser MAH, Blom CWPM (1993) Root porosities and radial oxygen losses of Rumex and Plantago species as influenced by soil pore diameter and soil aeration. New Phytol 125:565–574
Fleurbec (1987) Plantes sauvages des lacs, rivieres et tourbieres. Fleurbec, Saint-Augustin
Gebauer RLE, Reynolds JE, Tenhunen JD (1995) Growth and allocation of the arctic sedges Eriophorum angustifolium and E. vaginatum: effects of variable soil oxygen and nutrient availability. Oecologia 104:330–339
Gibbs J, Turner DW, Armstrong W, Darwent MJ, Greenway H (1998) Response to oxygen deficiency in primary maize roots. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Aust J Plant Physiol 25:745–758
Grime JP (2001) Plant strategies, vegetation processes and ecosystem properties. Wiley, Chichester
Grimoldi AA, Insausti P, Vasellati V, Striker GG (2005) Constitutive and plastic root traits and their role in differential tolerance to soil flooding among coexisting species of a lowland grassland. Int J Plant Sci 166:805–813
Hussner A, Meyer C, Busch J (2008) The influence of water level and nutrient availability on growth and root system development of Myriophyllum aquaticum. Weed Res 49:73–80
Insalud N, Bell RW, Colmer TD, Berkasem B (2006) Morphological and physiological responses of rice (Oryza sativa) to limited phosphorus supply in aerated and stagnant solution culture. Ann Bot 98:995–1004
Insausti P, Grimoldi AA, Chaneton EJ, Vasellati V (2001) Flooding induces a suite of adaptative plastic responses in the grass Paspalum dilatatum. New Phytol 152:291–299
Jackson MB, Armstrong W (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1:274–287
Jaeger C, Gessler A, Biller S, Rennenberg H, Kreuzwieser J (2009) Differences in C metabolism of ash species and provenances as a consequence of root oxygen deprivation by waterlogging. J Exp Bot 60:4335–4345
Kamaluddin M, Zwiazek JJ (2001) Metabolic inhibition of root water flow in red-osier dogwood (Cornus stolonifera) seedlings. J Exp Bot 52:739–745
Laan P, Berrevoets M, Lythe S, Armstrong W, Blom CWPM (1989) Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. J Ecol 77:693–703
Laanbroek HJ (1990) Bacterial cycling of minerals that affect plant growth in waterlogged soils: a review. Aquat Bot 38:109–125
Lambers H, Steingröver E, Smakman G (1978) The significance of oxygen transport and of metabolic adaptation in flood-tolerance of Senecio species. Physiol Plant 43:277–281
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Matsui T, Tsuchiya T (2006) Root aerobic respiration and growth characteristics of three Typha species in response to hypoxia. Ecol Res 21:470–475
Miller RC, Zedler JB (2003) Responses of native and invasive wetland plants to hydroperiod and water depth. Plant Ecol 167:57–69
Naidoo G, Naidoo S (1992) Waterlogging responses of Sporobolus virginicus (L.) Kunth. Oecologia 90:445–450
Puijalon S, Lena J-P, Bornette G (2007) Interactive effects of nutrient and mechanical stresses on plant morphology. Ann Bot 100:1297–1305
Rubio G, Oesterheld M, Alvarez CR, Lavado RS (1997) Mechanisms for the increase in phosphorus uptake of waterlogged plants: soil phosphorus availability, root morphology and uptake kinetics. Oecologia 112:150–155
Ryser P (2006) The mysterious root length. Plant Soil 286:1–6
Ryser P, Bernardi J, Merla A (2008) Determination of leaf fresh mass after storage between moist paper towels: constraints and reliability of the method. J Exp Bot 59:2461–2467
Ryser P, Emerson P (2007) Growth, root and leaf structure, and biomass allocation in Leucanthemum vulgare Lam. (Asteraceae) as influenced by heavy-metal-containing slag. Plant Soil 301:315–324
Schat H (1984) A comparative ecophysiological study on the effects of waterlogging and submergence on dune slack plants: growth, survival and mineral nutrition in sand culture experiments. Oecologia 62:279–286
Seago JL Jr, Peterson CA, Enstone DE, Scholey C (1999) Development of the endodermis and hypodermis of Typha glauca Godr. and Typha angustifolia L. roots. Can J Bot 77:122–134
Sorrell BK, Mendelssohn IA, McKee KL, Woods RA (2000) Ecophysiology of wetland plant roots: A modelling comparison of aeration in relation to species distribution. Ann Bot 86:675–685
Soukupowa L (1994) Allocation plasticity and modular structure in clonal graminoids in response to waterlogging. Folia Geobot Phytotx 29:227–236
Tennant D (1975) A test of a modified line intersect method of estimating root length. J Ecol 63:995–1001
Turner N (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58:339–366
van der Werf A, Kooijman A, Welschen R, Lambers H (1988) Respiratory energy costs for the maintenance of biomass, for growth and for ion uptake in roots of Carex diandra and Carex acutiformis. Physiol Plant 72:483–491
Vasellati V, Oesterheld M, Medan D, Loreti J (2001) Effects of flooding and drought on the anatomy of Paspalum dilatatum. Ann Bot 88:355–360
Vernescu C, Ryser P (2009) Constraints on leaf structural traits in wetland plants. Am J Bot 96:1068–1074
Visser E, Bögemann G (2003) Measurement of porosity in very small samples of plant tissue. Plant Soil 253:81–90
Visser EJW, Bögemann GM, Van de Steeg HM, Pierik R, Blom CWPM (2000a) Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol 148:93–103
Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ (2000b) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ 23:1237–1245
Voesenek LACJ, Blom CWPM, Pouwels RHW (1989) Root and shoot development of Rumex species under waterlogged conditions. Can J Bot 67:1865–1869
Wahl S, Ryser P (2000) Root tissue structure is linked to ecological strategies of grasses. New Phytol 148:459–471
Wahl S, Ryser P, Edwards PJ (2001) Phenotypic plasticity of grass root anatomy in response to light intensity and nutrient supply. Ann Bot 88:1071–1078
Zhang Y, Lin X, Werner W (2003) The effect of soil flooding on the transformation of Fe oxides and the adsorption/description behavior of phosphate. J Plant Nutr Soil Sc 166:68–75
Acknowledgements
The research was supported by an NSERC Discovery Grant (#249689) and an NSERC Undergraduate Student Research Award (HKG). We thank Céline Boudreau-Larivière for the use of her cryostat, and Ewa Cholewa for anatomical advice and constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric J.W. Visser.
Rights and permissions
About this article
Cite this article
Ryser, P., Gill, H.K. & Byrne, C.J. Constraints of root response to waterlogging in Alisma triviale . Plant Soil 343, 247–260 (2011). https://doi.org/10.1007/s11104-011-0715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0715-0