Abstract
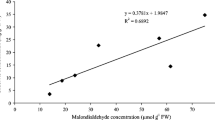
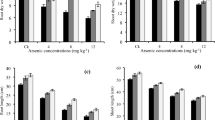
Arsenic-induced oxidative stress in chickpea was investigated under glasshouse conditions in response to application of arsenic and phosphorus. Three levels of arsenic (0, 30 and 60 mg kg−1) and four levels of P (50, 100, 200, and 400 mg kg−1) were applied to soil-grown plants. Increasing levels of both arsenic and P significantly increased arsenic concentrations in the plants. Shoot growth was reduced with increased arsenic supply regardless of applied P levels. Applied arsenic induced oxidative stress in the plants, and the concentrations of H2O2 and lipid peroxidation were increased. Activity of superoxide dismutase (SOD) and concentrations of non-enzymatic antioxidants decreased in these plants, but activities of catalase (CAT) and ascorbate peroxidase (APX) were significantly increased under arsenic phytotoxicity. Increased supply of P decreased activities of CAT and APX, and decreased concentrations of non-enzymatic antioxidants, but the high-P plants had lowered lipid peroxidation. It can be concluded that P increased uptake of arsenic from the soil, probably by making it more available, but although plant growth was inhibited by arsenic the P may have partially protected the membranes from arsenic-induced oxidative stress.



Similar content being viewed by others
Abbreviations
- AA:
-
Non enzymatic antioxidant activity
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- PEDXRF:
-
Polarized energy dispersive X-ray fluorescence
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- XRF:
-
X-ray fluorescence
References
Anjos MJ, Lopes RT, Jesus EFO, Simabuco SM, Cesareo R (2002) Quantitative determination of metals in radish using X-ray fluorescence spectrometry. X-ray Spectrom 31:120–123
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinated wheat seeds. J Exp Bot 44:127–132
Carbonell-Barrachina AA, Burlo F, Valero D, Lopez E, Martinez-Romero D, Martinez-Sanchez F (1999) Arsenic toxicity and accumulation in turnip as affected by As chemical speciation. J Agric Food Chem 47:2288–2294
Castillo-Michel H, Parsons JG, Peralta-Videa JR, Martinez-Martinez A, Dokken KM, Gardea-Torresdey JL (2007) Use of X-ray absorption spectroscopy and biochemical techniques to characterize arsenic uptake and reduction in pea (Pisum sativum) plants. Plant Physiol Biochem 45:457–463
Chuparina EV, Gunicheva TN (2003) Nondestructive X-ray fluorescence determination of some elements in plant material. J Anal Chem 58:856–861
Fayiga AO, Ma LQ (2006) Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyperaccumulator Pteris vittata. Sci Total Environ 359:17–25
Gao Y, Mucci A (2001) Acid base reactions, phosphate and arsenate complexation, and their competitive adsorption at the surface of goethite in 0.7 M NaCl solution. Geochim Cosmochim Acta 65:2361–2378
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Gunes A, Inal A, Bagci EG, Pilbeam DJ (2007) Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant Soil 290:103–114
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24:713–722
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hodson MJ, Sangster AG (2002) X-ray microanalytical studies of mineral localization in the needles of white pine (Pinus strobus L.). Ann Bot (Lond) 89:367–374
Ivanova J, Djingova R, Kuleff I (1999) Possibilities of ED-XRF with radionuclide sources for analysis of plants. J Radioanal Nucl Chem 242:569–575
Jain M, Mathur G, Koul S, Sarin NB (2001) Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Rep 20:463–468
Meharg AA (1994) Integrated tolerance mechanisms—constitutive and adaptive plant responses to elevated metal concentrations in the environment. Plant Cell Environ 17:989–993
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and non resistant plant species. New Phytol 154:29–43
Meharg AA, Macnair MR (1992) Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43:519–524
Meharg AA, Macnair MR (1994) Relationship between plant phosphorus status and the kinetics of arsenate influx in clones of Deschampsia cespitosa (L.) Beauv that differ in their tolerance to arsenate. Plant Soil 162:99–106
Meharg AA, Naylor J, Macnair MR (1994) Phosphorus nutrition of arsenate-tolerant and nontolerant phenotypes of velvetgrass. J Environ Qual 23:234–238
Mishra A, Choudhuri MA (1999) Effect of salicylic acid on heavy-metal induced membrane deterioration mediated by lipoxygenase in rice. Biol Plant 42:409–415
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Mylona PV, Polidoros AN, Scandalios JG (1998) Modulation of antioxidant responses by arsenic in maize. Free Radic Biol Med 25:576–585
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139
Ochiai EI (1995) Toxicity of heavy metals and biological defense. J Chem Educ 72:479–484
Peryea FJ (1991) Phosphate-induced release of arsenic from soils contaminated with lead arsenate. Soil Sci Soc Am J 55:1301–1306
Pickering IJ, Prince RC, George MJ, Smith RD, George GN, Salt DE (2000) Reduction and coordination of arsenic in Indian mustard. Plant Physiol 122:1171–1178
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Requejo R, Tena M (2006) Maize response to arsenic toxicity as revealed by proteome analysis of plant shoots. Proteomics 6:156–162
Sairam RK, Srivastava GC, Agarwal S, Meena RC (2005) Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–91
Smith E, Naidu R, Alston AM (2002) Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorption. J Environ Qual 31:557–563
Smith PG, Koch I, Reimer KJ (2008) Uptake, transport and transformation of arsenate in radishes (Raphanus sativus). Sci Total Environ 390:188–197
Srivastava M, Ma LQ, Singh N, Singh S (2005) Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J Exp Bot 56:1332–1342
Stephens WE, Calder A (2004) Analysis of non-organic elements in plant foliage using polarised X-ray fluorescence spectrometry. Anal Chim Acta 527:89–96
Stoeva N, Berova M, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Stoeva N, Bineva T (2003) Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. Bulg J Plant Physiol 29:87–95
Tu S, Ma LQ, MacDonald GE, Bondada B (2004) Effects of arsenic species and phosphorus on arsenic absorption, arsenate reduction and thiol formation in excised parts of Pteris vittata L. Environ Exp Bot 51:121–131
Tu C, Ma LQ (2003) Effects of arsenate and phosphate on their accumulation by an arsenic-hyperaccumulator Pteris vittata L. Plant Soil 249:373–382
Tu C, Ma LQ (2004) Comparison of arsenic and phosphate uptake and distribution in arsenic hyperaccumulating and nonhyperaccumulating fern. J Plant Nutr 27:1227–1242
Ullrich-Eberius CI, Sanz A, Novacky AJ (1989) Evaluation of arsenate- and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba G1. J Exp Bot 40:119–128
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: A.C. Borstlap.
Rights and permissions
About this article
Cite this article
Gunes, A., Pilbeam, D.J. & Inal, A. Effect of arsenic–phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil 314, 211–220 (2009). https://doi.org/10.1007/s11104-008-9719-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9719-9