Abstract
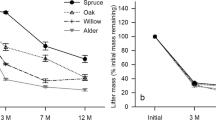
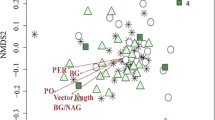
This paper tests whether individual trees in a mature forest stand influence the process of litter decomposition and the macroinvertebrate communities in the soil underneath their canopies, as a result of species-specific characteristics. A field decomposition experiment was performed in a mature forest stand of tropical montane cloud forest in Mexico. The areas under the canopies of Quercus laurina Humbl. & Bompl., Oreopanax xalapensis (Kunth) Decne. & Planchon and Beilschmedia ovalis (Blake) C. K. Allen trees were used as experimental units. The natural soil and litter macroinvertebrate communities were monitored and compared to the community that invaded decomposition boxes with reciprocally transplanted leaf litter. The abundances of four macroinvertebrate taxa in natural litter differed among tree species independently of season. No differences were found in the soil community. The response to experimental litter by macroinvertebrate taxa suggests that the production of a specific quality of litter is an important mechanism by which a tree influences the litter macroinvertebrate community that develops under its canopy. However, not all differences in community composition naturally found between tree species can be explained by differences in litter quality during the first year of decomposition. Differences in nutrient release that occur after the first year, and physical properties of litter also probably play an important role. Independently of the canopy tree, the initial chemical quality (N, P, Ca, Mg and lignin) of experimental litter largely determined the decomposition rate and nutrient dynamics of decomposing leaves. However, it was found that under O. xalapensis trees the breakdown of lignin from the litter produced by the same species of tree was particularly effective. This suggests that a feedback has developed between this tree species and the decomposer community prevailing under its canopy.




Similar content being viewed by others
References
Allan JE (1971) The preparation of agricultural samples for analysis by atomic absorption spectroscopy. Varian Techtron, Walnut Creek, p 15
Anderson JM (1975) Succession, diversity and trophic relationships of some soil animals in decomposing leaf litter. Ecology 44:475–495
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility: a handbook of methods. C.A.B. International, Wallingford, pp 1–221
Anonymous (1999) Estadístico del Estado de Oaxaca. Instituto Nacional de Geografía e Informática, Aguascalientes, Mexico
Ayres E, Dromph KM, Bardgett RD (2006) Do plant species encourage soil biota that specialise in the rapid decomposition of their litter? Soil Biol Biochem 38:183–186
Bardgett RD, Mawdsley JL, Edwards S, Hobbs PJ, Rodwell JS, Davies WJ (1999) Plant species and nitrogen effects on soil biological properties of temperate upland grassland. Funct Ecol 13:650–660
Bautista-Cruz A, del Castillo RF (2005) Soil changes during secondary succession in a tropical montane cloud forest area. Soil Sci Soc Am J 69:906–914
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Blanco-Macias AM (2001) Análisis sucesional del bosque mesófilo de montaña en El Rincón, Sierra Norte de Oaxaca. Facultad de Estudios Superiores Iztacala, UNAM, Mexico, pp 1–62
Boettcher SE, Kalisz PJ (1990) Single-tree influence on soil properties in the mountains of eastern Kentucky. Ecology 71:1365–1372
Conn C, Dighton J (2000) Litter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biol Biochem 32:489–496
Coûteaux M-M, Mousseau M, Célérier M-L, Bottner P (1991) Increased atmospheric CO2 and litter quality: decomposition of sweet chestnut leaf litter with animal food webs of different complexity. Oikos 61:54–64
Crawley MJ (2005) Statistics. An introduction using R. Wiley, Sussex, p 327
del Castillo RF (1996) Aspectos autoecológicos de Pinus chiapensis. In: Garduño LL, Chavarria GV, Magdaleno PL, Pérez IM (eds) Memorias del 2do. Coloquio Regional de Investigación, Ciencias Exactas y Naturales, Universidad Autónoma del Estado de México, Toluca, Estado de México.. Universidad Autónoma del Estado de México, Toluca, pp 63–68
Dijkstra FA (2003) Calcium mineralization in forest floor and surface soil beneath tree species in the northeastern US. For Ecol Manage 175:185–195
Ettema CH, Wardle DA (2002) Spatial soil ecology. Trends Ecol Evol 17:177–183
Görres JH, Dichiaro MJ, Lyons JB, Amador JA (1998) Spatial and temporal patterns of soil biological activity in a forest and an old field. Soil Biol Biochem 30:219–230
Guevara R, Romero I (2007) Buttressed trees of Brosimum alicatrum Sw. affect mycelial mat abundance and indirectly the composition of soil meso-fauna.. Soil Biol Biochem 39:289–294
Halaj J, Wise DH (2002) Impact of a detrital subsidy on the trophic cascade in a terrestrial grazing food web. Ecology 83:3141–3151
Hansen RA, Coleman DC (1998) Litter complexity and composition are determinant of the diversity and species composition of oribatid mites (Acari: Oribatid) in litterbags. Appl Soil Ecol 9:17–23
Hendrick RL, Pregitzer KS (1996) Temporal and depth-related patterns of fine root dynamics in northern hardwood forest. J Ecol 84:167–176
Kaye JP, Hart SC (1997) Competition for nitrogen between plants and soil microorganisms. Trends Ecol Evol 12:139–143
Kourtev PS, Ehrenfeld JG, Häggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecology 83:3152–3166
Lavelle P, Blanchart E, Martin A, Martin S, Spain A, Toutain F, Barois I, Schaefer R (1993) A hierarchical model for decomposition in terrestrial ecosystems: application to soils of the humid tropics. Biotropica 25:130–150
Lavelle P, Decaens T, Aubert M, Barot S, Blouin M, Bureau F, Margerie P, Mora P, Rossi JP (2006) Soil invertebrates and ecosystem services. Eur J Soil Biol 42:S3–S15
Migge S, Maraun M, Scheu S, Schaefer M (1998) The oribatid mite community (Acarina) of pure and mixed stands of beech (Fagus sylvatica) and spruce (Picea abies) of different age. Appl Soil Ecol 9:115–121
Negrete-Yankelevich S, Fragoso C, Newton AC, Heal OW (2006) Spatial patchiness of litter, nutrients and macroinvertebrates during secondary succession in a Tropical Montane Cloud Forest. Plant Soil 286:123–139
Negrete-Yankelevich S, Fragoso C, Newton AC (2007a) The impact of logging and secondary succession on the below-ground system of a cloud forest in Mexico. In: Newton AC (ed) Biodiversity loss and conservation in fragmented forest landscapes. Evidence from tropical Montane and South Temperate rain forests in Latin America. CABI International, Oxfordshire, pp 181–199
Negrete-Yankelevich S, Fragoso C, Newton AC, Heal OW (2007b) Successional changes in soil, litter and macroinvertebrate parameters following selective logging in a Mexican Cloud Forest. Appl Soil Ecol 35:340–355
Phillips JD, Marion DA (2004) Pedological memory in forest soil development. For Ecol Manage 188:363–380
Salamon JA, Alphei J, Ruf A, Schaefer M, Scheu S, Schneider K, Suhrig A, Maraun M (2006) Transitory dynamic effects in the soil invertebrate community in a temperate deciduous forest: Effects of resource quality. Soil Biol Biochem 38:209–221
Santos FP, Phillips J, Whitford WG (1981) The role of mites and nematodes in early stages of buried litter decomposition in a desert. Ecology 62:664–669
Scheu S, Schaffer M (1998) Bottom-up control of the soil macrofauna community in a beechwood limestone: manipulation of a food resource. Ecology 79:1573–1585
Stohlgren TJ (1988) Litter dynamics in two Sierra mixed conifer forests. II. Nutrient release in decomposing leaf litter.. Can J Bot 18:1136–1144
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Blackwell, Oxford, pp 1–372
Turner DP, Franz EH (1985) The influence of western hemlock and western red cedar on microbial numbers, nitrogen mineralization, and nitrification. Plant Soil 88:259–267
Van Soest PJ (1994) Fiber and physicochemical properties of feeds. In: Van Soest PJ (ed) Nutritional ecology of the ruminant. Cornell University Press, Ithaca, pp 140–160
Vohland K, Schroth G (1999) Distribution patterns of the litter macrofauna in agroforestry and monoculture plantations in central Amazonia as affected by plant species and management. Appl Soil Ecol 13:57–68
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in the soil. Biol Rev 67:321–358
Wardle DA, Bonner KI, Barker GM (2002) Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores. Funct Ecol 16:585–595
Wardle DA, Yeates GW, Williamson W, Bonner KI (2003) The response of three trophic level soil food web to the identity and diversity of plant species and functional groups. Oikos 102:45–56
Warren MW, Zou X (2002) Soil macrofauna and litter nutrients in three tropical tree plantations on a disturbed site in Puerto Rico. For Ecol Manage 170:161–171
Whelan MJ, Anderson JM (1996) Modelling spatial patterns of throughfall and interception loss in a Norway spruce (Picea abies) plantation at the plot scale. J Hydrol 186:335–354
Widden P (1985) Microfungal community structure from forest soils in southern Quebec, using discriminant function and factor analysis. Can J Bot-Rev Can Bot 64:1402–1412
Zinke PJ (1962) The pattern of influence of individual forests trees on soil properties. Ecology 43:130–133
Acknowledgements
This research was funded by a postgraduate scholarship provided by the Mexican Consejo Nacional de Ciencia y Tecnología (Num. Reg. 131536) and by supplementary support by The UK Darwin Initiative. We are indebted to Jo Anderson, David Wardle, Vinicio Sosa, Roger Guevara, Carolina Valdespino and Rafael del Castillo for invaluable suggestions during the development of this research. We are particularly grateful for field assistance by Raúl Rivera and dedicated illustration by Rafael Ruiz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tibor Kalapos.
Rights and permissions
About this article
Cite this article
Negrete-Yankelevich, S., Fragoso, C., Newton, A.C. et al. Species-specific characteristics of trees can determine the litter macroinvertebrate community and decomposition process below their canopies. Plant Soil 307, 83–97 (2008). https://doi.org/10.1007/s11104-008-9585-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9585-5