Abstract
In intensive horticultural crops, the choice of growing media and the adequate management of irrigation must ensure an optimal trade-off between aeration and water supply to roots. The proportion of gas-filled pores and their composition can be strongly affected by the water status and hence by irrigation. In this context, continuous measurement of gas exchange and water status of the growing medium could bring out some insights into how irrigation events affect root activity and aeration in a time scale of minutes to several hours. For this purpose, a measuring system was developed that measured the CO2 efflux rate from the entire substrate root system of pot plants while their shoots were kept outside, undisturbed. It was able to monitor four plants at a time for several weeks at a rate of one measurement per plant every 10 min, thus tracing the dynamics of CO2 efflux through a great many irrigation cycles. The results showed a marked pattern of CO2 efflux around each irrigation event, consisting mainly of a sharp, conspicuous peak followed by a depression until a threshold in substrate water potential was reached. Analysis of these data suggests that the pattern is imposed mainly by the effects of irrigation and water content on the mobility of gases in the growing medium. The peak can be explained by the CO2-enriched air being displaced by the water added to the growing medium in the pot, and the following depression can be the result of the reduced mobility of gases when substrate water content is high. In spite of the great variation in the instantaneous efflux rate of CO2, the integration of these CO2 values for the entire day provides a rather predictable value given the root biomass and does not seem to be affected by the number of irrigation events that occur in a given day.





Similar content being viewed by others
Abbreviations
- CER:
-
CO2 efflux rate
- RDW:
-
root dry weight
- SWP:
-
substrate water potential
References
Allaire SE, Caron J, Duchesne I, Parent LE, Rioux JA (1996) Air-filled porosity, gas relative diffusivity, and tortuosity: Indices of Prunus x cistena sp growth in peat substrates. J Am Soc Hortic Sci 121:236–242
Allaire-Leung SE, Caron J, Parent LE (1999) Changes in physical properties of peat substrates during plant growth. Can J Soil Sci 79:137–139
Argo WR, Biernbaum JA, Fonteno WC (1996) Root medium carbon dioxide and oxygen partial pressures for container-grown chrysanthemums. Hortscience 31:385–388
Baggs EM (2006) Partitioning the components of soil respiration: a research challenge. Plant Soil 284:1–5
Bhattarai SP, Su NH, Midmore DJ (2005) Oxygation unlocks yield potentials of crops in oxygen-limited soil environments. Adv Agron 88:313
Boone RD, Nadelhoffer KJ, Canary JD, Kaye JP (1998) Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 396:570–572
Bottner P (1985) Response of microbial biomass to alternate moist and dry conditions in a soil incubated with C-14-labelled and N-15-labelled plant-material. Soil Biol Biochem 17:329–337
Bouma TJ, Bryla DR (2000) On the assessment of root and soil respiration for soils of different textures: interactions with soil moisture contents and soil CO2 concentrations. Plant Soil 227:215–221
Bouma TJ, Nielsen KL, Eissenstat DM, Lynch JP (1997) Estimating respiration of roots in soil: Interactions with soil CO2, soil temperature and soil water content. Plant Soil 195:221–232
Bowden RD, Newkirk KM, Rullo GM (1998) Carbon dioxide and methane fluxes by a forest soil under laboratory-controlled moisture and temperature conditions. Soil Biol Biochem 30:1591–1597
Buchmann N (2000) Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol Biochem 32:1625–1635
Bunt AC (1988) Media and mixes for container-grown plants, 2nd edn. Unwin Hyman, London
Campbell GS (1988) Soil physics with basic. Elsevier, New York
Carazo N, López D, Mancilla S, Martínez A, Cáceres R, Marfà O (2005) Oxifertigation and foliar nutrient contents of closed soilless rose crop. Acta Hortic 697:493–497
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Chang Biol 4:217–227
Glinski J, Stepniewski W (1985) Soil aeration and its role for plants. CRC, Boca Raton
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48:115–146
Hodge A, Paterson E, Thornton B, Millard P, Killham K (1997) Effects of photon flux density on carbon partitioning and rhizosphere carbon flow of Lolium perenne. J Exp Bot 48:1797–1805
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Hogberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Huang BR, North GB, Nobel PS (1993) Soil seaths, photosynthate distribution to roots, and rhizosphere water relations for Opuntia-Ficus-indica. I J Plant Sci 154:425–431
Jarvis PG (1995) The role of temperate trees and forests in CO2 fixation. Vegetation 121:157–174
Jones SB, Or D (1998) Design of porous media for optimal gas and liquid fluxes to plant roots. Soil Sci Soc Am J 62:563–573
Kieft TL, Soroker E, Firestone MK (1987) Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biol Biochem 19:119–126
King JA, Smith KA (1987) Gaseous-diffusion through peat. J Soil Sci 38:173–177
Kucera CL, Kirkham DR (1971) Soil respiration studies in tallgrass prairie in Missouri. Ecology 52:912–915
Kuzyakov Y, Cheng W (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33:1915–1925
Kuzyakov Y, Cheng W (2004) Photosynthesis controls of CO2 efflux from maize rhizosphere. Plant Soil 263:85–99
Lee MS, Nakane K, Nakatsubo T, Mo WH, Koizumi H (2002) Effects of rainfall events on soil CO2 flux in a cool temperate deciduous broad-leaved forest. Ecol Res 17:401–409
Lee X, Wu HJ, Sigler J, Oishi C, Siccama T (2004) Rapid and transient response of soil respiration to rain. Glob Chang Biol 10:1017–1026
Lemaire F, Rivière L, Stievenard S, Marfa O, Gschwander S, Giuffrida F (1998) Consequences of organic matter biodregradability on the physical, chemical parameters of substrates. Acta Hortic 469:129–138
Lloyd J, Taylor JA (1994) On the temperature-dependence of soil respiration. Funct Ecol 8:315–323
Marfà O, Martínez A, Orozco R, Serrano L, Martínez FX (1992) The use of fine-grade perlites in lettuce bag cultures. II. Physical properties, rheologic effects and productivity. Acta Hortic 342:339–348
Marfà O, Cáceres R, Guri S (2005) Oxyfertirrigation: a new technique for soilless culture under mediterranean conditions. Acta Hortic 697:65–72
Milks RR, Fonteno WC, Larson RA (1989) Hydrology of horticultural substrates. 2. Predicting physical-properties of media in containers. J Am Soc Hortic Sci 114:53–56
Morard P, Silvestre J (1996) Plant injury due to oxygen deficiency in the root environment of soilless culture: a review. Plant Soil 184:243–254
Orozco R (1995) Propiedades físicas e hidrológicas de perlitas utilizadas para cultivos sin suelo. Su implicación con las relaciones sustrato-agua-planta y con el riego de cultivos hortícolas. Universitat de Lleida, Lleida (Spain)
Orozco R, Marfà O (1995) Granulometric alteration, air-entry potential and hydraulic conductivity in perlites used in soilless cultures. Acta Hortic 408:147–161
Penman HL (1940) Gas and vapor movements in soil: the diffusion of vapors through porous solids. J Agric Sci 30:437–462
Poorter H, Vanderwerf A, Atkin OK, Lambers H (1991) Respiratory energy-requirements of roots vary with the potential growth-rate of a plant-species. Physiol Plant 83:469–475
Raich JW, Schlesinger WH (1992) The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus Ser B Chem Phys Meteorol 44:81–99
Reth S, Reichstein M, Falge E (2005) The effect of soil water content, soil temperature, soil pH-value and the root mass on soil CO2 efflux – a modified model. Plant Soil 268:21–33
Rivière LM, Foucard JC, Lemaire F (1990) Irrigation of container crops according to the substrate. Sci Hortic 43:339–349
Rivière LM, Charpentier S, Jeannin B, Kafka B (1993) Oxygen concentration of nutrient solution in mineral wools. Acta Hortic 342:93–101
Rochette P, Desjardins RL, Pattey E (1991) Spatial and temporal variability of soil respiration in agricultural fields. Can J Soil Sci 71:189–196
Samartzidis C, Awada T, Maloupa E, Radogiou K, Constantinidou HIA (2005) Rose productivity and physiological responses to different substrates for soil-less culture. Sci Hortic 106:203–212
Silber A, Xu G, Levkovitch I, Soriano S, Bilu A, Wallach R (2003) High fertigation frequency: the effects on uptake of nutrients, water and plant growth. Plant Soil 253:467–477
Smart DR, Peñuelas J (2005) Short-term CO2 emissions from planted soil subject to elevated CO2 and simulated precipitation. Appl Soil Ecol 28:247–257
Strojny Z, Nelson, PV, Willitz, RM (1998) Pot soil air composition in conditions of high soil moisture and its influence on chrysanthemum growth. Sci Hortic 73:125–136
Urrestarazu M, Mazuela PC (2005) Effect of slow-release oxygen supply by fertigation on horticultural crops under soilless culture. Sci Hortic 106:484–490
Warembourg FR, Paul EA (1977) Seasonal transfers of assimilated C-14 in grassland – plant production and turnover, soil and plant respiration. Soil Biol Biochem 9:295–301
Xu LK, Baldocchi DD, Tang JW (2004) How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob Biogeochem Cycles 18: GB4002, DOI 10.1029/2004GB002281
Acknowledgements
This study was supported by the Spanish Comisión Interministerial de Ciencia y Tecnología (CICYT), project reference AGL2002-04098-C02-02. We would also like to thank Anna Puerta and José Montero for their help with the technical work in the laboratory and in the field, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Per Ambus.
Proposed cover photograph
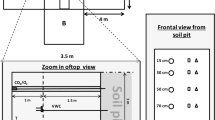
Illustration of a rose plant installed in its cuvette. The substrate root system is enclosed in the cuvette for the measuring period while the shoot is kept undisturbed outside. The container is placed in another container that collects the drainage and discharges it through a siphon, thus allowing irrigation to be performed during uninterrupted monitoring of CO2 exchange.
Rights and permissions
About this article
Cite this article
Casadesus, J., Caceres, R. & Marfa, O. Dynamics of CO2 efflux from the substrate root system of container-grown plants associated with irrigation cycles. Plant Soil 300, 71–82 (2007). https://doi.org/10.1007/s11104-007-9389-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9389-z