Abstract
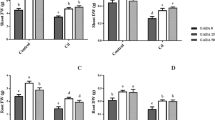
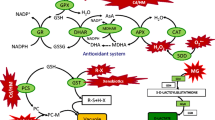
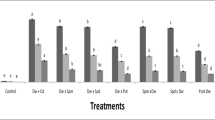
The protective effect of polyamines against Cd toxicity of rice (Oryza sativa) leaves was investigated. Cd toxicity to rice leaves was determined by the decrease in protein content. CdCl2 treatment results in (1) increased Cd content, (2) induction of Cd toxicity, (3) increase in H2O2 and malondialdehyde (MDA) contents, (4) decrease in ascorbic acid (ASC) and reduced glutathione (GSH) contents, and (5) increase in the activities of antioxidative enzymes (superoxide dismutase, glutathione reductase, ascorbate peroxidase, catalase, and peroxidase). Spermidine (Spd) and spermine (Spm), but not putrescine (Put), were effective in reducing CdCl2-induced toxicity. Spd and Spm prevented CdCl2-induced increase in the contents of H2O2 and MDA, decrease in the contents of ASC and GSH, and increase in the activities of antioxidative enzymes. Spd and Spm pretreatments resulted in a decrease in Cd content when compared with H2O pretreatment, indicating that Spd and Spm may reduce the uptake of Cd. Results of the present study suggest that Spd and Spm are able to protect Cd-induced oxidative damage and this protection is most likely related to the avoidance of H2O2 generation and the reduction of Cd uptake.









Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbic aicd
- CAT:
-
Catalase
- DAB:
-
3,3′-Diaminobenzidine
- DHA:
-
Dehydroascorbate
- DW:
-
Dry weight
- FW:
-
Fresh weight
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- POX:
-
Peroxidase
- Put:
-
Putrescine
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- Spd:
-
Spermidine
- Spm:
-
Spermine
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 85:235–241
Asada K (1999) The water–water cycle in chloroplasts: scavenging of reactive oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:501–639
Benavides MP, Gallego SM, Comga MZ, Tomaro ML (2000) Relationship between polyamines and paraquat toxicity in sunflower leaf discs. Plant Growth Regul 31:215–224
Borrell A, Carbonell L, Farrás R, Puig-Paellada P, Tiburcio AF (1997) Polyamines inhibit lipid peroxidation in senescing oat leaves. Physiol Plant 99:385–390
Bors W, Langebartels C, Michel C, Sandermann H (1989) Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry 28:1589–1595
Bouchereau A, Aziz A, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang CJ, Kao CH (1997) Paraquat toxicity is reduced by polyamines in rice leaves. Plant Growth Regul 22:163–168
Chaoui A, Mazhoudi S, Ghorbal MH, Ferjani EE (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Chen CT, Kao CH (1991) Senescence of rice leaves. XXX. Levels of endogenous polyamines and dark-induced senescence of rice leaves. Plant Cell Physiol 32:935–941
Chen SL, Kao CH (1995) Cd induced changes in proline level and peroxidase activity in roots of rice seedlings. Plant Growth Regul 17:67–71
Chien HF, Kao CH (2000) Accumulation of ammonium in rice leaves in response to excess cadmium. Plant Sci 156:111–115
Chien HF, Lin CC, Wang JW, Chen CT, Kao CH (2002) Changes in ammonium ion content and glutamine synthetase activity in rice leaves caused by excess cadmium are a consequence of oxidative damage. Plant Growth Regul 36:41–47
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Das P, Sammantaray S, Rout GR (1997) Studies on cadmium toxicity: a review. Environ Pollut 98:29–36
Dixit V, Pandey V, Shyam R (2001) Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot 52:1101–1109
Drolet G, Dumbroff EB, Legge RL, Thompson JE (1986) Radical scavenging properties of polyamines. Phytochemistry 25:367–371
Foster JG, Hess JL (1980) Response of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487
Foyer CH, Descourvies P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studies in transgenic plants. Plant Cell Environ 17:507–523
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
Foyer CH, Noctor G (2005) Oxidant and antioxidant signaling in plants: re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071
Gallego SM, Benavides MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Groppa MD, Tomaro ML, Benavides MP (2001) Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci 161:481–488
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hou SM, Kao CH (1993) Characteristics of the induction of the ethylene by cadmium in detached rice leaves. Bot Bull Acad Sin 34:163–168
Hsu YT, Kao CH (2003) Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 26:867–874
Hsu YT, Kao CH (2005) Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol Plant 124:71–80
Innelli MA, Pietrni R, Fiore L, Petrilli L, Massacci A (2002) Antioxidant response to cadmium in Phragmites anstrals plants. Plant Physiol Biochem 40:977–982
Jana S, Choudhuri MA (1982) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Krupa Z (1988) Cadmium-induced changes in the composition and structure of the light-harvesting chlorophyll a/b protein complex II in radish cotyledons. Physiol Plant 73:518–524
Kuo MC, Kao CH (2004) Antioxidant enzyme activities are upregulated in response to cadmium in sensitive, but not in tolerant rice (Oryza sativa L.) seedlings. Bot Bull Acad Sin 45:291–299
Kurepa J, Smalle J, Van Montagu M, Inzé D (1998) Polyamines and paraquat toxicity in Arabidopsis thaliana. Plant Cell Physiol 39:987–992
Larsson EH, Bordman JF, Asp H (1998) Influence of UV-B radiation and Cd2+ on chlorophyll fluorescence, growth and nutrient content in Brassica napus. J Exp Bot 49:1031–1039
León AM, Palma JM, Corpas FJ, Gomez M, Romeropuertas MC, Chatterjee D, Mateos RM, del Rio LA, Sandalio LM (2002) Antioxidative enzymes in cultivars of pepper plants with different sensitivity to cadmium. Plant Phsyiol Biochem 40:813–820
Li C-Z, Wang G-X (2004) Interaction between reactive oxygen species, ethylene and polyamines in leaves of Glycyrrhiza inflata seedlings under root osmotic stress. Plant Growth Regul 42:55–60
Lozano-Rodriguez E, Hernández LE, Bonay P, Carpena-Ruiz R (1997) Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 48:123–128
MacAdam JW, Nelson CJ, Sharpe RE (1992) Peroxidase activity in the leaf elongation zone of tall fescue. Plant Physiol 99:872–878
Minton KW, Tabor H, Tabor CW (1990) Paraquat toxicity is increased in Escherichia coli defective in the synthesis of polyamines. Proc Natl Acad Sci USA 87:2851–2855
Nakano Y, Asda K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:807–880
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Olmos EO, Martínez-Solano JR, Piqueras A, Hellín E (2003) Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot 54:291–301
Ormrod DP, Beckerson DW (1986) Polyamines as antiozonants for tomato. HortScience 21:1070–1071
Orozco-Cárdenas ML, Ryan CA (1999) Hydrogen peroxide is generated systematically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96:6553–6557
Orozco-Cárdenas M, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13:179–191
Paoletti F, Aldinucci D, Mocali A, Capparini A (1986) A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem 154:536–541
Piqueras A, Olmos E, Martínez-Solano JR, Hellín E (1999) Cd induced oxidative burst in tobacco BY-2 cell: time-course, subcellular location and antioxidant response. Free Radical Res 31:S25–S31
Romero-Puertas MC, Rodriguez-Serrano M, Corpas FJ, Gómez M, del Rio LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O2- and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Romero-Puertas MC, Zablza A, Rodriguez-Serrano M, Gómez M, del Rio LA, Sandalio LM (2003) Antioxidative response to cadmium in pea roots. Free Radical Res 37:44
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plant. J Exp Bot 52:2115–2126
Schützendübel A, Polle A (2002) Plant responses to biotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1366
Schützendübel A, Schwang P, Teichmann T, Gross K, Langenfeld-Heyer R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol 127:887–898
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Sharma SS, Dietz K-J (2006) The significance of amino acids and amino acid-derived molecules in plant response and adaptation to heavy metal stress. J Exp Bot 57:711–726
Shaw BP (1995) Effect of mercury and cadmium on the activities of antioxidative enzymes in the seedlings of Phaseolus aureus. Biol Plant 37:587–596
Siedlecka A, Baszynski T (1993) Inhibition of electron flow around photosystem I in chloroplasts of cadmium-treated maize plants is due to cadmium-induced iron deficiency. Physiol Plant 87:199–202
Slocum RD, Kaur-Sawhney R, Galston AW (1984) The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys 235:283–303
Stobart AK, Griffiths WT, Ameen-Bukhari I, Sherwood RP (1985) The effect of Cd2+ on the biosynthesis of chlorophyll of barley. Physiol Plant 63:293–298
Tang W, Newton RJ, Outhavong V (2004) Exogenously added polyamines recover browning tissues into normal callus cultures and improve plant regeneration in pine. Physiol Plant 122:386–395
Thompson JE, Legge RL, Barber RF (1987) The role of free radicals in senescence and wounding. New Phytol 105:317–344
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 151:59–66
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 5:173–212
Wallace HM, Fraser AV, Hughes A (2003) A perspective of polyamines metabolism. Biochem J 376:1–14
Weinstein LH, Kaur-Sawhney R, Rajam MV, Wettlaufer SH, Galston AW (1986) Cadmium-induced accumulation of putrescine in oat and bean leaves. Plant Physiol 82:641–645
Yu YB, Yang SF (1980) Biosynthesis of wound ethylene. Plant Physiol 66:281–285
Acknowledgements
This work was supported by a research grant from the National Science Council of the Republic of China (NSC 95-2313-B002-007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, Y.T., Kao, C.H. Cadmium-induced oxidative damage in rice leaves is reduced by polyamines. Plant Soil 291, 27–37 (2007). https://doi.org/10.1007/s11104-006-9171-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9171-7