Abstract
Diamine putrescine (Put) and polyamines; spermidine (Spd) and spermine (Spm) are essential component of every cell because of their involvement in the regulation of cell division, growth and development. The aim of this study is to enhance the levels of Put during fruit development and see its implications in ripening and quality of tomato fruits. Transgenic tomato plants over-expressing mouse ornithine decarboxylase gene under the control of fruit-specific promoter (2A11) were developed. Transgenic fruits exhibited enhanced levels of Put, Spd and Spm, with a concomitant reduction in ethylene levels, rate of respiration and physiological loss of water. Consequently such fruits displayed significant delay of on-vine ripening and prolonged shelf life over untransformed fruits. The activation of Put biosynthetic pathway at the onset of ripening in transgenic fruits is also consistent with the improvement of qualitative traits such as total soluble solids, titratable acids and total sugars. Such changes were associated with alteration in expression pattern of ripening specific genes. Transgenic fruits were also fortified with important nutraceuticals like lycopene, ascorbate and antioxidants. Therefore, these transgenic tomatoes would be useful for the improvement of tomato cultivars through breeding approaches.





Similar content being viewed by others
References
Alcázar R, Altabella T, Marco F, Bortolotii C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Apelbaum A, Burgoon AC, Anderson JD, Lieberman M, Benarie R, Mattoo AK (1981) Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplast. Plant Physiol 68:453–456
Bajaj S, Rajam MV (1996) Polyamine accumulation and near loss of morphogenesis in long term Callus cultures of rice: restoration of plant regeneration by manipulation of cellular polyamine levels. Plant Physiol 112:1343–1348
Bassie L, Zhu C, Romagosa I, Christou P, Capell T (2007) Transgenic wheat plants expressing an oat arginine decarboxylase cDNA exhibit increases in polyamine content in vegetative tissue and seeds. Mol Breed. doi:10.1007/s11032-007-9154-2
Berta G, Altamura M, Fusconi A, Cerruti F, Capitani F, Bagni N (1997) The plant cell wall is altered by inhibition of polyamine biosynthesis. New Phytol 137:569–577
Brummell DA (2006) Cell wall disassembly in ripening fruit. Funct Plant Biol 33:103–119
Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P (1999) Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 11:2203–2216
Cassol T, Mattoo AK (2002) Do polyamines and ethylene interact to regulate plant growth, development and senescence? In: Nath P, Mattoo A, Ranade SR, Weil JH (eds) Molecular insights in plant biology. Science Publishers, Enfield, pp 121–132
Defilippi BG, Dandekar AM, Kader AA (2004) Impact of suppression of ethylene action or biosynthesis on flavor metabolites in apple (Malus domestica Borkh) fruits. J Agric Food Chem 52:5694–5701
D’Orazi D, Bagni N (1987) In vitro interactions between polyamines and pectic substances. Biochem Biophys Res Commun 148:1259–1263
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fanasca S, Colla G, Maiani G, Venneria E, Rouphael Y, Azzini E, Saccardo F (2006) Changes in antioxidant content of tomato fruits in response to cultivar and nutrient solution composition. J Agric Food Chem 54:4319–4325
Ferguson IB, Boyd LM (2002) Inorganic nutrients and fruit quality. In: Knee M (ed) Fruit quality and its biological basis. Sheffield Academic Press, Sheffield, pp 14–45
Giovannoni JJ (2001) Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 52:725–749
Guillén F, Castillo S, Zapata PJ, Martínez-Romero D, Serrano M, Valero D (2007) Efficacy of 1-MCP treatment in tomato fruit. 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol Technol 43:23–27
Gupta A, Pal RK, Rajam MV (2013) Delayed ripening and improved fruit processing quality in tomato by RNAi-mediated silencing of three homologs of 1-aminopropane-1-carboxylate synthase gene. J Plant Physiol 170:987–995
Hamilton AJ, Lycett GW, Grierson D (1990) Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 346:284–287
Handa AK, Mattoo AK (2010) Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol Biochem 48:540–546
Hobbs SLA, Warkentin TD, DeLong CMO (1993) Transgene copy number can be positively or negatively associated with transgene expression. Plant Mol Biol 21:17–26
Igarashi K, Kashiwagi K (2010) Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42:39–51
Kameji T, Pegg AE (1987) Effect of putrescine on the synthesis of S-adenosylmethioine decarboxylase. Biochem J 243:285–288
Khan AS, Singh Z, Abbasi NA, Swinny EE (2008) Pre- or postharvest applications of putrescine and low temperature storage affect fruit ripening and quality of ‘Angelino’ plum. J Sci Food Agric 88:1686–1695
Knee M, Bartley IM (1981) In: Friend J, Rhodes MJC (eds) Recent advances in the biochemistry of fruit and vegetables. Academic Press, New York, pp 131–148
Kumar SV, Rajam MV (2004) Polyamine-ethylene nexus: a potential target for postharvest biotechnology. Indian J Biotech 3:299–304
Kumar A, Taylor MA, Arif SAM, Davies HV (1996) Potato plants expressing antisense and sense S-adenosylmethionine decarboxylase (SAMDC) transgenes show altered levels of polyamines and ethylene: antisense plants display abnormal phenotypes. Plant J 9:147–158
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381
Kushad MM, Dumbroff EB (1991) Metabolic and physiological relationship between the polyamine and ethylene biosynthetic pathways. In: Slocum RD, Flores HE (eds) Biochemistry and physiology of polyamines in plants. CRC Press, Boca Raton, pp 77–92
Lasanajak Y, Minocha R, Minocha SC, Goyal R, Fatima T, Handa AK, Mattoo AK (2014) Enhanced flux of substrates into polyamine biosynthesis but not ethylene in tomato fruit engineered with yeast S-adenosylmethionine decarboxylase gene. Amino Acids 46:729–742
Lashbrook CC, Giovannoni JJ, Hall BD, Fischer RL, Bennett AB (1998) Transgenic analysis of tomato endo-beta-1,4-glucanase gene function. Role of cel1 in floral abscission. Plant J 13:303–310
Li N, Parsons B, Liu D, Mattoo AK (1992) Accumulation of wound-inducible ACC synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol Biol 18:477–487
Madhulatha P, Pandey R, Hazarika P, Rajam MV (2007) High transformation frequency in Agrobacterium mediated genetic transformation of tomato by using polyamines and maltose in shoot regeneration medium. Physiol Mol Biol Plants 13:191–198
Madhulatha P, Gupta A, Gupta S, Kumar A, Pal RK, Rajam MV (2014) Fruit-specific over-expression of human S-adenosylmethionine decarboxylase gene results in polyamine accumulation and affects diverse aspects of tomato fruit development and quality. J Plant Biochem Biotechnol 23:151–160
Mehta R, Cassol T, Li N, Ali N, Handa AK, Mattoo AK (2002) Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotech 20:613–618
Mirdehghan SH, Rahemi M, Castillo S, Martínez-Romero D, Serrano M, Valero D (2007) Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharvest Biol Technol 44:26–33
Nambeesan S, Datsenka T, Ferruzzi MG, Malladi A, Mattoo AK, Handa AK (2010) Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J 63:836–847
Neily MH, Matsukura C, Maucourt M, Bernillon S, Deborde C, Moing A, Yina Y, Saitoa T, Moria K, Asamizua E, Rolinb D, Moriguchie T, Ezuraa H (2011) Enhanced polyamine accumulation alters carotenoid metabolism at the transcriptional level in tomato fruit over-expressing spermidine synthase. J Plant Physiol 168:242–252
Oeller PW, Lu MW, Taylor LP, Pike DA, Theologis A (1991) Reversible inhibition of tomato fruit senescence by antisense RNA. Science 254:437–439
Oke M, Pinhero RG, Paliyath G (2003) The effects of genetic transformation of tomato with antisense phospholipase D cDNA on the quality characteristics of fruits and their processed products. Food Biotechnol 17:163–182
Parra-Lobato MC, Gomez-Jimenez MC (2011) Polyamine-induced modulation of genes involved in ethylene biosynthesis and signaling pathways and nitric oxide production during olive mature fruit abscission. J Exp Bot 62:4447–4465
Payasi A, Sanwal GG (2010) Ripening of climacteric fruits and their control. J Food Biochem 34:679–710
Pear JR, Ridge N, Rasmussen R, Rose RE, Houck CM (1989) Isolation and characterization of a fruit-specific cDNA and the corresponding clone from tomato. Plant Mol Biol 13:639–651
Pirrello J, Regad F, Latché A, Pech JC, Bouzayen M (2009) Regulation of tomato fruit ripening. In: Perspectives in agriculture, veterinary science, nutrition and natural resources. CAB Rev 4:1–14
Ranganna S (1977) Manual of analysis of fruit and vegetable products. Tata McGraw Hill, New Delhi
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbour, New York
Sauter M, Moffatt B, Saechao MC, Hell R, Wirtz M (2013) Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis. Biochem J 451:145–154
Schwartz CE, Wang X, Stevenson RE, Pegg AE (2011) Spermine synthase deficiency resulting in X-linked intellectual disability (Snyder–Robinson syndrome). Methods Mol Biol 720:437–445
Serrano M, Martinez-Romero D, Guillén F, Valero D (2003) Effects of exogenous putrescine on improving shelf life of four plum cultivars. Postharvest Biol Technol 30:259–271
Singh SP, Pal RK (2008) Controlled atmosphere storage of guava (Psidium guajava L.) fruit. Postharvest Biol Technol 47:296–306
Sinha R, Rajam MV (2013) RNAi silencing of three homologues of S-adenosylmethionine decarboxylase gene in tapetal tissue of tomato results in male sterility. Plant Mol Biol 82:169–180
Srivastava RP, Kumar S (1993) Fruit and vegetable preservation—principles and practices. International book distribution company, New Delhi
Takahashi T, Kakehi J-I (2010) Polyamines: ubiquitous polycations with unique roles in growth and stress Responses. Ann Bot 105:1–6
Torrigiani P, Bressanin D, Beatriz RK, Tadiello A, Trainotti L, Bonghi C, Ziosi V, Costa G (2012) Spermidine application to young developing peach fruits leads to a slowing down of ripening by impairing ripening-related ethylene and auxin metabolism and signalling. Plant Physiol 146:86–98
Valero D, Martínez-Romero D, Serrano M (2002) The role of polyamines in the improvement of the shelf life of fruit. Trends Food Sci Technol 13:228–232
Wang T-W, Zhang C-G, Wu W, Nowack LM, Madey E, Thompson JE (2005) Antisense suppression of deoxyhypusine synthase in tomato delays fruit softening and alters growth and development. Plant Physiol 138:1372–1382
Watada AE, Aulenbach BB, Worthington JT (1976) Vitamins A and C in ripe tomatoes as affected by stage of ripeness at harvest and by supplementary ethylene. J Food Sci 41:856–858
Winer L, Apelbaum A (1986) Involvement of polyamines in the development and ripening of avocado fruits. J Plant Physiol 126:223–456
Xie Q, Hu Z, Zhu Z, Dong T, Zhao Z, Cui B, Chen G (2014) Overexpression of a novel MADS-box gene SlFYFL delays senescence, fruit ripening and abscission in tomato. Sci Rep. doi:10.1038/srep04367
Xiong AS, Yao QH, Peng RH, Li X, Han PL, Fan HQ (2005) Different effects on ACC oxidase gene silencing triggered by RNA interference in transgenic tomato. Plant Cell Rep 23:639–646
Yahia EM, Contreras-Padilla M, Gonzalez-Aguilar G (2001) Ascorbic acid content in relation to ascorbic acid oxidase and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. Lebensm-Wiss Technol 34:452–457
Ziosi V, Bregoli AM, Bonghi C, Fossati T, Biondi S, Costa G, Torrigiani P (2006) Transcription of ethylene perception and biosynthesis genes is altered by putrescine, spermidine and aminoethoxyvinylglycine (AVG) during ripening in peach fruit (Prunus persica). New Phytol 172:229–238
Acknowledgments
We express our gratitude to Prof. Tony Pegg (Hershey, USA) for providing the m-ODC cDNA and Dr. V. S. Reddy (International Centre for Genetic Engineering and Biotechnology, New Delhi) for providing the 2A11 tomato promoter. This work was generously supported by Grants from the Department of Biotechnology (Govt. of India), New Delhi (Grant Nos. BT/PR/2990/Agr/16/232/2002 and BT/PR8657/PBD/16/738/2007). We also thank the University Grants Commission, New Delhi for Special Assistance Programme and Department of Science and Technology, New Delhi for FIST and DU-DST PURSE programmes. Research fellowships to Roopali Pandey and Aarti Gupta by the Council of Scientific and Industrial Research are acknowledged.
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Roopali Pandey and Aarti Gupta have contributed equally to the article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2014_273_MOESM2_ESM.jpg
Supplementary Fig. 2 Molecular characterization and segregation analysis of tomato transformants. (a) PCR analysis with primers specific to NPT-II gene in primary transformants: L - 1 kb ladder; PC - Plasmid DNA; UT - DNA from untransformed control; LeODC 22-72 - DNA from different transgenic tomato lines. (b) Southern blot analysis of T0 generation of LeODC transgenic plants, revealing transgene integration and copy number using m-ODC gene radio-labeled probe. UT - DNA from untransformed control; LeODC 11-60- DNA from different transgenic lines digested with Xba I enzyme. (c) The leaves of T1 transgenic line LeODC22 showing segregation of the m-ODC transgene on kanamycin (50 mg/l) amended MS basal medium, and this was based on the bleaching of leaves. The untransformed leaves (UT) showed complete bleaching on antibiotic medium. (d1 and d2). PCR analysis with primers specific to m-ODC gene showing segregation pattern of transgene in T2 and T3 generations respectively. L - 1 kb ladder; PC - Plasmid DNA, UT-DNA from untransformed control; B - blank, 1-12 - DNA from different progenies of LeODC27 line. (e) Southern blot analysis of T3 generation of LeODC transgenic plants revealing homozygous status of integrated transgene, using m-ODC gene radio-labeled probe. LeODC22-LeODC32 - DNA from T3 progenies of different transgenic lines digested with XbaI enzyme. (JPEG 90 kb)
11103_2014_273_MOESM3_ESM.jpg
Supplementary Fig. 3 A lineage chart illustrating analysis of segregation patterns across different generations. Leaves from 100 T1 seedlings (named as e.g., LeODC22-1, LeODC22-2……. LeODC22-100) of each positive T0 plant (e.g. LeODC22) were screened by kanamycin resistant assay and 40 positive segregants were further confirmed by m-ODC gene specific PCR. All the homozygous lines followed 3:1 transgene segregation pattern in T1 generation. Ten positive segregants were taken to raise T2 generation. Hundred T2 seedlings (further named as e.g., LeODC22-1.1, LeODC22-1.2……. LeODC22-1.100.) from each of 10 such parents were further screened by kanamycin resistance assay and confirmed by PCR. (JPEG 72 kb)
11103_2014_273_MOESM4_ESM.jpg
Supplementary Fig. 4 Pollen viability UT and transgenic tomato lines. Pollen viability was scored based on pollen germination after 2 h incubation in germination media in dark. Data is the representation of T3 generation showing means of 500 pollen counts. Each experiment was repeated thrice in four generations (T0-T3) with reproducible results. (JPEG 12 kb)
11103_2014_273_MOESM5_ESM.jpg
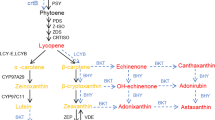
Supplementary Fig. 5 Semi-quantitative RT-PCR analysis showing expression patterns of fruit specific genes. (a) Expression pattern of PA biosynthesis genes; SlODC, SlADC, SlSAMDC1 and SlSPDSYN, (b) Expression pattern of ethylene biosynthesis genes; SlACS2, SlACS4 and SlACO1, (c) Expression pattern of cell wall hydrolyzing genes; SlEXP1, SlTBG4, SlPG2a and SlXTH (d) Expression pattern of lycopene biosynthesis genes; SlPSY1, SlDXS1 and SlLES; in transgenic (T3 generation) and UT fruits at different stages (MG, BR, PR and RR) of ripening. SlACTIN gene expression was maintained as internal control. Expression analysis was done thrice using 50 ng of total RNA, with ‘n’ no. of cycles utilizing three biological replicates. (JPEG 69 kb)
Rights and permissions
About this article
Cite this article
Pandey, R., Gupta, A., Chowdhary, A. et al. Over-expression of mouse ornithine decarboxylase gene under the control of fruit-specific promoter enhances fruit quality in tomato. Plant Mol Biol 87, 249–260 (2015). https://doi.org/10.1007/s11103-014-0273-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-014-0273-y