Abstract
In the endoplasmic reticulum, immature polypeptides coincide with terminally misfolded proteins. Consequently, cells need a well-balanced quality control system, which decides about the fate of individual proteins and maintains protein homeostasis. Misfolded and unassembled proteins are sent for destruction via the endoplasmic reticulum-associated degradation (ERAD) machinery to prevent the accumulation of potentially toxic protein aggregates. Here, we report the identification of Arabidopsis thaliana OS9 as a component of the plant ERAD pathway. OS9 is an ER-resident glycoprotein containing a mannose-6-phosphate receptor homology domain, which is also found in yeast and mammalian lectins involved in ERAD. OS9 fused to the C-terminal domain of YOS9 can complement the ERAD defect of the corresponding yeast Δyos9 mutant. An A. thaliana OS9 loss-of-function line suppresses the severe growth phenotype of the bri1-5 and bri1-9 mutant plants, which harbour mutated forms of the brassinosteroid receptor BRI1. Co-immunoprecipitation studies demonstrated that OS9 associates with Arabidopsis SEL1L/HRD3, which is part of the plant ERAD complex and with the ERAD substrates BRI1-5 and BRI1-9, but only the binding to BRI1-5 occurs in a glycan-dependent way. OS9-deficiency results in activation of the unfolded protein response and reduces salt tolerance, highlighting the role of OS9 during ER stress. We propose that OS9 is a component of the plant ERAD machinery and may act specifically in the glycoprotein degradation pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In all eukaryotes, the endoplasmic reticulum (ER) is the site of folding, maturation and assembly of proteins entering the secretory pathway. In the ER these soluble and membrane-bound proteins attain their native conformation before they are delivered to different compartments of the endomembrane system and to the extracellular space. Folding and maturation of the proteins is guided by ER-resident chaperones, lectins and enzymes that work together in a protein quality control system to ensure that only correctly folded and assembled proteins are released to the Golgi (Ellgaard and Helenius 2003). To maintain the protein homeostasis in the ER terminally misfolded proteins or proteins that do not integrate into cognate complexes are subjected to degradation in a tightly regulated multistep process referred to as ER-associated degradation (ERAD) (McCracken and Brodsky 1996). In this process non-native proteins are recognized, retrotranslocated to the cytosol, ubiquitinated and finally destroyed by the 26S proteasome (Vembar and Brodsky 2008).
Many proteins of the secretory pathway are modified with N-linked oligosaccharides, which are added mainly co-translationally upon entry of the nascent polypeptide chain into the ER. The attached N-glycans can have a direct impact on the folding process of proteins or they indirectly affect folding by subjecting polypeptides to the calnexin/calreticulin cycle (Helenius and Aebi 2001). Moreover, specific N-glycan structures on aberrant proteins play a crucial role for ERAD. For yeast it has been demonstrated that the Man7GlcNAc2 oligosaccharide, which is generated by the subsequent action of two class I α-mannosidases, exposes a terminal α1,6-linked mannosyl-residue that marks glycoproteins for degradation by ERAD (Clerc et al. 2009). This specific mannose residue is recognized by the ER-resident lectin YOS9, which acts as a quality control receptor and links the recognition of glycoproteins destined for degradation to the ubiquitin-proteasome system (Quan et al. 2008; Clerc et al. 2009).
YOS9 and its mammalian homologs OS-9 and XTP3-B contain mannose-6-phosphate receptor homology domains (MRH) and mutations in the conserved carbohydrate-binding sites reduce the rate of misfolded glycoprotein breakdown (Bhamidipati et al. 2005; Szathmary et al. 2005; Hosokawa et al. 2009). In the current model for yeast ERAD, YOS9 interacts with the ER membrane-embedded HRD3-HRD1 ubiquitin ligase complex. The luminal domain of HRD3 recognizes misfolded polypeptide segments of aberrant proteins and YOS9 scans them for the presence of the glycan signal, leading to retrotranslocation of the ERAD substrates to the cytoplasm, polyubiquitinylation by the HRD1 E3 ubiquitin-ligase and proteasomal degradation. Analogous to this model, the mammalian OS-9 and XTP3-B proteins interact with the SEL1L-HRD1 complex and play a crucial role for ERAD of glycoproteins. In contrast to the well-characterized yeast and mammalian ERAD pathways comparatively little is known about the process in plants, especially the glycan-dependent steps remain elusive (Vitale and Boston 2008). Earlier studies reported on the degradation of non-glycosylated proteins (Brandizzi et al. 2003; Müller et al. 2005) or on glycoproteins, where the glycosylation status did not play a specific role for the degradation of the substrates (Marshall et al. 2008). The first indication that glycan trimming reactions are involved in the degradation of misfolded proteins in plants came from the analysis of defective forms of the Arabidopsis brassinosteroid receptor BRI1 (Hong et al. 2008, 2009). BRI1 is a plasma membrane located leucine-rich repeat receptor like kinase that contains 14 putative N-glycosylation sites in its ectodomain (Li and Chory 1997). The mutant BRI1 forms BRI1-9 (S662F) and BRI1-5 (C69Y) were found to be retained in the ER by quality control mechanisms. Treatment with the specific class I α-mannosidase inhibitor kifunensine (Elbein et al. 1990) stabilized the mutated BRI1 forms and suppressed the characteristic growth phenotype of the bri1-5 and bri1-9 mutants indicating that BRI1-5 and BRI1-9 are subjected to ERAD (Jin et al. 2007; Hong et al. 2008, 2009). In Arabidopsis kifunensine specifically inhibits α-mannosidases (MNS proteins) involved in N-glycan trimming (Liebminger et al. 2009) and mutants which are deficient in the ALG12 α1,6-mannosyltransferase and lack the specific α1,6-mannosyl residue are defective in degradation of mutated BRI1-5 and BRI1-9 (Hong et al. 2009). These results highlight that a similar glycan-dependent ERAD pathway is functional in plants, which involves removal of mannose residues from distinct oligosaccharides and recognition of a specific glycan signal by an ER-resident lectin similar to YOS9 and OS-9/XTP3-B.
To identify proteins involved in ERAD of plant glycoproteins we analyzed an Arabidopsis protein with a MRH-domain for its role in the degradation of the glycoprotein ERAD substrates BRI1-5 and BRI1-9. Here, we describe the involvement of Arabidopsis OS9 in ERAD and we show that OS9 deficiency activates the unfolded protein response (UPR) and results in increased susceptibility to salt stress.
Materials and methods
Plant materials
Arabidopsis thaliana Col-0 is the ecotype for all mutants, except for bri1-6 (En-2 ecotype) and bri1-9 (Ws-2 ecotype). All plants were grown under long-day conditions at 22°C as described previously (Liebminger et al. 2009). T-DNA insertion lines os9-1 (SALK_029413), sel1l (SALK_109430), bri1-6 and bri1-5 bak1-1D were obtained from the European Arabidopsis Stock Centre (http://arabidopsis.info). The bri1-9 seeds were a kind gift of Frans E. Tax. To obtain the bri1-5 mutant, bri1-5 bak1-1D was backcrossed to Col-0 and the absence of the T-DNA in the BAK1 locus (Li et al. 2002) was confirmed by genotyping. For treatments with NaCl, KCl, tunicamycin (Sigma–Aldrich, http://www.sigmaaldrich.com) and paraquat (Sigma–Aldrich) seedlings were grown on Murashige and Skoog (MS) medium as indicated. Nicotiana benthamiana plants were grown under long-day condition at 24°C.
PCR genotyping
For os9-1, the T-DNA insertion was confirmed by PCR using the left border primer LBa1 and the gene-specific primer At5g35080_1F and LBa1/At5g35080_2R (Table S1). Homozygous os9-1 plants were identified by screening with primers At5g35080_1F/_2R and homozygous sel1l lines were identified with primers LBa1/At1g18260_1F and At1g18260_1F/_2R. bri1-5, bri1-6 and bri1-9 were crossed with os9-1 and the double mutants were identified by PCR genotyping and DNA sequencing. To genotype bri1-5, a part of the BRI1 locus was amplified with primers BRI1_15F/_16R. For confirmation of the bri1-6 mutation, primers BRI1_10F/_14R were used for amplification, while for bri1-9 primers BRI1_13F/_14R were used.
Reverse transcription-PCR
RNA isolation and cDNA synthesis was done as described previously (Strasser et al. 2007). The OS9 coding region including a part of the 5′- and 3′-untranslated regions was amplified using primers At5g35080_6F/_7R. To detect OS9 transcripts, PCR was performed from cDNA using the primers At5g35080_3F/_2R.
Plasmid construction and generation of transgenic plants
To create C-terminal fusion proteins, the OS9 coding region was amplified from cDNA by PCR with primers At5g35080_4F/_5R and ligated into XbaI/BamHI digested plasmids p20F (Strasser et al. 2007), p20F-Fc (Schoberer et al. 2009) and p31 (like p20F but mRFP instead of GFP) resulting in the binary plant expression vectors p20-AtOS9-GFP (35S:OS9-GFP), p20-AtOS9-Fc-GFP (35S:OS9-GFPglyc) and p31-AtOS9-mRFP (35S:OS9-mRFP), respectively. OS9 containing the R201A mutation was generated by site-directed mutagenesis of p20-AtOS9-Fc-GFP using the Quikchange II kit (Stratagene, http://genomics.agilent.com/) with the primers At5g35080_23F/_23R. For complementation of the os9-1 mutant, wild type, os9-1 and os9-1 bri1-5 plants were floral dipped with Agrobacterium tumefaciens strain UIA143 containing p20-AtOS9-GFP, p20-AtOS9-Fc-GFP and p20-AtOS9R201A-Fc-GFP, respectively, and transgenic plants were selected on MS plates containing 50 μg mL−1 kanamycin.
For generation of transgenic plants expressing BRI1 with a C-terminal myc-tag, the BRI1 coding region was amplified from cDNA with primers BRI1_1F/_2R, BamHI/NcoI-digested and ligated into BamHI-linearized pPT8 (Strasser et al. 2006) to create the binary plant expression vector pPT8-BRI1-myc. The vector pPT8-bri1-9-myc was created by site-directed mutagenesis using primers BRI1_7F/_7R. pPT8-bri1-5-myc was created by swapping the N-terminal part of BRI1 in pPT8-BRI1-myc with the N-terminal part of bri1-5 (obtained by amplification with primers BRI1_17F/_18R) by making use of the internal XbaI restriction site. BRI1-GFP and BRI1-5-GFP were generated in the same way by cloning of the BRI1 fragments into p20F. The plasmid expressing a C-terminal HA-tagged SEL1L (p60SEL1L) was generated by amplification of the SEL1L open reading frame with primers At1g18260_10F/_11R. The product was subcloned, SpeI/BglII-digested and ligated into XbaI/BamHI-linearized p60 plasmid, which is a derivative of p20F that contains a 3× HA-tag sequence instead of GFP.
Subcellular localization of OS9-GFP
Transient expression in leaves of 4–5 week-old N. benthamiana plants was done by infiltration of a suspension of the A. tumefaciens strain UIA143 (OD600 of 0.1–0.2) containing p20-AtOS9-GFP, p20-AtOS9-Fc-GFP or p31-AtOS9-mRFP. Sections of infiltrated leaves were analysed 1–2 days after infiltration on a Leica TCS SP2 confocal microscope as described recently (Schoberer et al. 2009). For co-localization, agrobacteria containing the ER/Golgi-marker GnTI-CaaaTS-mRFP (Schoberer et al. 2009) were co-infiltrated with p20-AtOS9-GFP or p20-AtOS9-Fc-GFP.
Co-purification
For co-purification experiments, leaves of 4–5 week-old N. benthamiana plants were co-infiltrated with agrobacteria (OD600 of 0.2–0.3) containing p20-AtOS9-Fc-GFP or p20-AtOS9R201A-Fc-GFP (both contain a domain from the IgG heavy chain which binds very efficiently to Protein A) and either pPT8-BRI1-myc, pPT8-bri1-5-myc, pPT8-bri1-9-myc or p60SEL1L. Proteins were extracted from 1 g of leaves with RIPA buffer (Sigma–Aldrich) supplemented with 1% (v/v) protease inhibitor cocktail (Sigma–Aldrich), followed by incubation on ice for 30 min and centrifugation at 10,000g for 10 min at 4°C. The cleared supernatant was incubated with 40 μL rProtein A-Sepharose (GE Healthcare, http://www.gelifesciences.com) for 1 h at 4°C on a rocking shaker and washed extensively with RIPA buffer on a Micro Bio-Spin Chromatography column (Bio-Rad, http://www.bio-rad.com). Finally, the protein fractions were eluted by boiling in 1× SDS sample buffer, separated by 7% SDS–PAGE and analyzed by immunoblotting using rabbit polyclonal anti-myc (A-14, Santa Curz Biotechnology, http://www.scbt.com), mouse monoclonal anti-HA (12CA5) or anti-BRI1 (custom-made in rabbits against the peptide: CGKRPTDSPDFGDNN, GenScript, http://www.genscript.com/) and anti-OS9 (custom-made in rabbits against the peptide: LPEDSPFHPGDNLEC, GenScript) antibodies.
Endo H treatment
Leaves from 4 to 6 week-old plants were frozen in liquid nitrogen, homogenized in a mixer mill and a crude protein extract obtained by adding 1.5× SDS sample buffer, followed by incubation on ice for 30 min. After centrifugation at 10,000g for 10 min at 4°C, the supernatant was denatured at 95°C for 10 min in 1× Denaturing Buffer (NEB, http://www.neb-online.de) and incubated with or without 1,000 U of Endo H (NEB) in 1× G5 buffer for 3 h at 37°C. The protein extracts were then analyzed by immunoblotting with anti-BRI1 or anti-myc antibodies.
Yeast growth conditions, transformation and complementation assays
The yeast BY4741 wild type (MATa; his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and Δyos9 (Y03993; Mata his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YDR057 yos9::kanMX4) strains are from the EUROSCARF collection. The complementation assay was performed using the yeast ERAD substrate CPY* as previously described (Jakob et al. 1998). Briefly, yeast strains were transformed with the BglII-linearized integrating plasmid pRS306-prc1-1 (Knop et al. 1996) followed by selection on plates containing 5-fluoroorotic acid. For screening of colonies, a fragment of the PRC1 locus was amplified with primers Sc_CPY_1F/_5R and positive clones were identified by resistance of the PCR fragment towards BstXI digestion. Sequencing of the PCR fragment with primer Sc_CPY_5R confirmed the G255R mutation of prc1-1. The yos9::kanMX4 disruption was confirmed by PCR with primers Sc_Yos9_1F/KanB, Sc_Yos9_2R/KanC, KanD/KanE and Sc_Yos9_1F/_2R.
For the yeast complementation assay, the vector pADHfw containing the ADH1 promoter and a LEU2 marker was used. As a positive control, the coding sequence of the yeast YOS9 gene was amplified by PCR with primers Sc_Yos9_7F/_8R, XhoI/BamHI-digested and ligated into XhoI/BamHI-digested pADHfw. The Arabidopsis OS9 coding sequence was amplified by PCR from cDNA with primers At5g35080_9F/_10R and cloned in the same way. The Arabidopsis OS9 coding sequence with a C-terminal HDEL motif was created by PCR from the plasmid p20-AtOS9-GFP with the primers At5g35080_22F/_21R, XhoI/BamHI-digested and ligated into pADHfw. For generation of the plasmid expressing the full-length OS9 sequence fused to the C-terminal part of YOS9, the OS9 open reading frame was amplified with primers At5g35080_22F/-rev-SalI, while the region encoding the C-terminal region of YOS9 was amplified from genomic yeast DNA with primers Sc_Yos9_8R/-fw-SalI. Both PCR fragments were subcloned, cut out by BamHI/SalI- and XhoI/SalI-digestion, respectively and ligated together by their compatible SalI sites. The ligation product was used as a template for PCR with primers At5g35080_22F/Sc_Yos9_8R, XhoI/BamHI-digested and ligated into pADHfw.
Analysis of CPY* degradation
To analyze the CPY* degradation, a cycloheximide assay was performed as described (Clerc et al. 2009). Briefly, yeast cells were grown at 30°C to mid-log phase (OD600 of 1.0–1.2) and the chase was started by addition of cycloheximide (200 μg mL−1; Sigma–Aldrich). 6 × 108 cells were removed at each time point (0, 40, 80 and 120 min), transferred to ice-cold NaN3 (10 mM) and harvested by centrifugation. After addition of 150 μL of ice-cold lysis buffer (50 mM Tris–HCl, pH 7.5, 1% SDS, 1 mM EDTA, 1 mM PMSF), the cells were disrupted by vigorous shaking for 2 min at 4°C with 100 μL glass beads. Proteins were subjected to 10% SDS–PAGE, followed by immunoblotting with anti-CPY antibody (Invitrogen). A ChemiDoc Imager (Bio-Rad) was used for signal detection and the Quantity One program (Version 4.6.9) for quantification.
Results
Identification of Arabidopsis MRH domain proteins
The Arabidopsis genome contains two MRH domain-containing proteins. One is annotated as the α-glucosidase II β-subunit, which is involved in removal of terminal α1,3-glucose residues during N-glycan trimming and the calnexin/calreticulin cycle (Quinn et al. 2009; Soussilane et al. 2009; D‘Alessio et al. 2010). The second Arabidopsis MRH domain protein (At5g35080) herein referred to as OS9 is a 282 amino acid long protein with a predicted signal peptide and three putative N-glycosylation sites (N94, N169, N190). The amino acid sequence of the OS9 MRH-region has 27% identity to YOS9 and 37 and 31% identity to human OS-9 and XTP3-B, respectively. In contrast to YOS9 the Arabidopsis OS9 protein does not contain a C-terminal HDEL motif (Fig. 1a) and the C-terminal domain that is present in YOS9 and human OS-9 (Hosokawa et al. 2010; Satoh et al. 2010) is completely missing in OS9. In contrast, the six cysteine residues involved in disulfide bridge formation and the residues of the carbohydrate binding site (Munro 2001; Satoh et al. 2010) are all conserved (Fig. 1c) suggesting that OS9 could be a mannose binding protein with a related function.
OS9 is a MRH domain protein that is ubiquitously expressed. a A comparison of the domain organization of OS9 with yeast (YOS9) and human (OS-9/XTP3-B) MRH domain proteins is shown. The signal peptide is indicated in grey, the MRH domain is shown in black. For OS-9 and XTP3-B the longest variants are shown (Hosokawa et al. 2010). b The chimeric OS9-YOS9 protein that was used for complementation of Δyos9 yeast consists of amino acids 1–272 from Arabidopsis OS9 fused to amino acids 277–542 from yeast YOS9. c Sequence alignment of MRH domains was performed using ClustalW (http://www.ebi.ac.uk/Tools/clustalw2/index.html). The domains were identified according to Munro (2001). Conserved residues are shaded (100% identity black, 80% identity dark grey, 60% identity light grey). The six conserved cysteines involved in the formation of disulphide bonds are marked in blue, amino acid residues involved in carbohydrate binding are marked in red (Roberts et al. 1998; Szathmary et al. 2005) and putative N-glycosylation sites are marked in yellow. The two tryptophan residues that determine the α1,6-mannose binding specificity of human OS-9 are marked in magenta (Satoh et al. 2010) and the three mannose binding residues of YOS9 are marked in green (Ghosh et al. 2003; Bhamidipati et al. 2005). d Proteins were extracted from different A. thaliana organs or developmental stages (RL rosette leaves, S whole seedlings, YL young leaves, R root, FL flower, SI siliques), separated by SDS–PAGE and analyzed by immunoblots using OS9-specific antibodies. α-tubulin expression was used as a control
To examine the expression pattern of OS9 in A. thaliana, protein extracts from different plant organs were subjected to SDS–PAGE and immunoblotting with an OS9-specific antibody. The intensity of the protein band was similar in all analyzed plant organs (Fig. 1d), which is consistent with DNA microarray data (https://www.genevestigator.com/), suggesting that OS9 is ubiquitously expressed during development. Co-expression analysis using ATTED-II (http://atted.jp/) indicated that OS9 is expressed together with ER-located chaperones like SHD, SDF2 and ERdj3B, which are involved in ER quality control processes in plants (Ishiguro et al. 2002; Yamamoto et al. 2008; Nekrasov et al. 2009; Schott et al. 2010).
OS9 is an ER-located glycoprotein
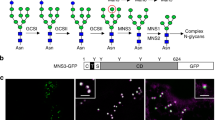
The absence of any transmembrane domain and known ER-retention/retrieval signals suggest that OS9 is secreted in plants. However, involvement in ERAD requires localization of OS9 in the ER. To determine its subcellular location we fused the fluorescent proteins GFPglyc (Schoberer et al. 2009) and mRFP, respectively, to the C-terminal end of OS9. The resulting OS9-mRFP and OS9-GFPglyc proteins were transiently expressed in N. benthamiana leaf epidermal cells and analyzed by confocal laser scanning microscopy. For both constructs a reticulate fluorescent pattern was observed indicating accumulation of the protein in the ER (Fig. 2a, b). Co-expression of OS9-GFPglyc and GnTI-CaaaTS-mRFP, a marker which predominantly labels the ER (Schoberer et al. 2009), showed an overlap of both fluorescent proteins (Fig. 2c). We purified OS9-GFPglyc from N. benthamiana leaves by affinity chromatography and analyzed peptides for the presence of N-glycans by LC–ESI–MS. All three N-glycosylation sites of OS9 were occupied with oligomannosidic N-glycans (Fig. S1). Consistently, endogenous OS9 from A. thaliana seedlings was sensitive to Endo H digestion. Together these data demonstrate that OS9 is located in the ER (Fig. 2d).
Fluorescent protein-tagged OS9 displays ER localization. Confocal microscopy of N. benthamiana leaf epidermal cells expressing a OS9-GFPglyc, b OS9-mRFP and c OS9-GFPglyc (in green) together with the ER-retained construct GnTI-CaaaTS-mRFP (in magenta) reveals ER localization of OS9 (merged image). Scale bars = 10 μM. d Immunoblot analysis of the glycosylation status of endogenous OS9. Samples were subjected to Endo H digestion, separated by SDS–PAGE and analyzed by immunoblotting with OS9-specific antibodies or α-tubulin as a control
A chimeric OS9-YOS9 fusion protein suppresses the yeast Δyos9 defect
A mutated form of the soluble carboxypeptidase Y (CPY*) protein serves as an ERAD substrate in yeast. In wild-type yeast CPY* is rapidly degraded, while deletion of YOS9 in the Δyos9 mutant reduces CPY* degradation (Szathmary et al. 2005). To test if Arabidopsis OS9 is the functional homolog of YOS9 we expressed it under a constitutive promoter in Δyos9 yeast cells expressing CPY* (Δyos9 CPY*). Although the Arabidopsis OS9 protein was expressed in yeast as determined by immunoblotting (data not shown) it did not result in significant degradation of CPY* (Fig. 3a, b). In contrast, the expression of YOS9, which served as a control, led to profound degradation of CPY* during the cycloheximide incubation period. This finding suggests that Arabidopsis OS9 cannot substitute for YOS9. Possible explanations could be that OS9 is not properly retained in the ER of S. cerevisiae since it does not contain a HDEL/KDEL retrieval signal, or that the C-terminal part of YOS9 is required for its function in yeast. To test these two possibilities we generated one HDEL-tagged OS9 form (OS9-HDEL) and a chimeric form composed of the C-terminal domain of YOS9 (amino acids 277–542) fused to OS9 (OS9-YOS9) (Fig. 1b). Whilst expression of OS9-HDEL did not result in an improved degradation of CPY* (Fig. 3a, b), OS9-YOS9 increased the degradation of CPY*. In summary, these results show that Arabidopsis OS9 cannot complement the CPY* degradation defect of the yeast Δyos9 mutant, because it lacks the C-terminal domain of YOS9, that is required for association with the membrane-embedded HRD3-HRD1 ubiquitin ligase complex (Gauss et al. 2006).
A chimeric OS9-YOS9 protein suppresses the yeast Δyos9 defect. a Equal cell numbers of yeast strains expressing the ERAD substrate CPY* and OS9 or YOS9 were incubated with cycloheximide and proteins were extracted at the given time points. Shown are representative immunoblot images. The lower panel shows a Ponceau S staining of the membrane. b Signal intensities (vertical axis) of the CPY* specific band from three independent experiments were quantified and blotted against time. The amount of the CPY* protein band at time point 0 was set to 100%
The UPR is activated in the os9-1 mutant
We searched in the mutant collections for putative OS9 knockout lines and identified the SALK_029413 line. We screened the segregating population and identified a homozygous T-DNA insertion line termed os9-1. One of the T-DNA insertion sites in os9-1 was located at an intron–exon junction (Fig. 4a) resulting in the formation of a smaller transcript derived from an incorrect splicing event (Fig. 4b). The aberrant transcript completely lacks exons 6 and 7 of the OS9 gene but contains a functional open reading frame leading to a protein with a 58 amino acid deletion. In addition we identified low amounts of another aberrant transcript in os9-1 which harbours a deletion of exon 6 resulting in the formation of a transcript with a premature stop codon. By immunoblotting a band corresponding to the full-length OS9 protein or truncated versions thereof could not be detected in the os9-1 mutant. Moreover, since the deletion of exons 6 and 7 affects almost half of the MRH domain, the os9-1 line very likely does not produce a functional OS9 protein and thus represents a null allele (Fig. 4c).
os9-1 lacks a functional OS9 protein and displays activation of the UPR. a Schematic overview of the OS9 gene structure. Boxes represent exons (the black area represents the coding region), the os9-1 T-DNA insertion is indicated. b Reverse transcription-PCR analysis of the os9-1 mutant using oligonucleotides that flank the insertion site. UBQ5 amplification served as a control. c Immunoblot with anti-OS9 antibodies. The asterisk indicates an unspecific band, which was used as a loading control. d Protein extracts from wt and os9-1 14-day-old seedlings were subjected to SDS–PAGE and Coomassie brilliant blue (CBB) staining. e Blots were analyzed using concanavalin A (ConA) (f) or anti-horseradish peroxidase (HRP) antibodies, which recognize N-glycans with β1,2-xylose and core α1,3-fucose residues. g Protein extracts were analyzed by immunoblotting using anti-PDI, anti-BiP2 and anti-TGG1 antibodies, respectively. h 10-day-old seedlings were incubated for 24 h in ½× MS medium supplemented with 3% sucrose and 5 μg mL−1 tunicamycin (TM), protein extracts were analyzed using OS9 or α-tubulin antibodies
To monitor whether OS9 deficiency results in altered N-glycosylation or differences in protein levels we extracted proteins from os9-1 leaves and subjected them to SDS-PAGE and immunoblotting. No proteins with altered migration were detected by Coomassie staining of protein extracts (Fig. 4d). The analysis using antibodies against complex N-glycans and lectin overlays with concanavalin A (ConA) did not reveal any drastic changes in the staining pattern (Fig. 4e, f). Total N-glycan analysis of protein extracts from leaves showed that the overall composition of N-glycans was similar to wild type in os9-1, with a minor increase of Man5GlcNAc2 structures and a decrease of truncated processed (MMXF) N-glycans (Fig. S2). These findings strongly indicate that OS9 is not directly involved in N-glycan processing.
Underglycosylation of particular proteins leads to a change in mobility when analyzed by SDS–PAGE. However, neither the ER-resident protein disulfide isomerase (PDI) nor the thioglucoside glucohydrolase TGG1, which have both been shown to be affected in mutants with an underglycosylation defect (Koiwa et al. 2003; Lerouxel et al. 2005; Farid et al. 2011), displayed altered mobility in os9-1 (Fig. 4g). However, PDI and binding protein 2 (BiP2) protein levels were increased in os9-1, indicating the activation of the UPR due to the absence of a functional OS9 protein.
We also tested whether the UPR increases OS9 protein levels. Seedlings were incubated with tunicamycin and OS9 expression was monitored by immunoblotting. A clear shift in mobility as well as upregulation of OS9 was observed in wild-type plants treated with tunicamycin (Fig. 4h) indicating that OS9 expression is induced by the UPR.
OS9 deficiency suppresses the dwarf phenotype of bri1-5 and bri1-9 mutants
It has been reported that the dwarf phenotype of bri1-5 can be rescued by kifunensine treatment or by mutants that block the ERAD pathway (Hong et al. 2009; Liu et al. 2011; Su et al. 2011). We suspected that OS9 could play a crucial role in ERAD of glycoproteins in plants similar to YOS9 and human OS-9/XTP3-B. To test this hypothesis we crossed os9-1 to bri1-5 and analyzed the double mutant for phenotypic changes. os9-1 was able to partially suppress the bri1-5 phenotype when seedlings were grown under light/dark growth conditions (Fig. S3) and almost completely restored the growth defect of plants cultivated on soil (Fig. 5a, b). Ectopic expression of OS9-GFP in os9-1 bri1-5 resulted in the generation of transgenic plants with the dwarf phenotype (Fig. 5c and Fig. S4) strongly indicating that a functional OS9 protein interferes with the maturation of BRI1-5.
os9-1 suppresses the bri1-5 phenotype by affecting the ER-retention of BRI1-5. a 17-day and b 4-week-old soil-grown plants. c Expression of OS9-GFP in os9-1 bri1-5 plants restores the bri1-5 growth phenotype. os9-1 bri1-5 double mutants were floral dipped with 35S:OS9-GFP. Expression of 35S:OS9-GFP in wild-type is shown as a control. d 10-week-old bri1-6 single and os9-1 bri1-6 double mutants display the bri1-6 dwarf phenotype
Apart from BRI1-5 it was also shown that BRI1-9, another mutated BRI1 form, is retained in the ER and only released to the plasma membrane upon blockage of the ERAD pathway or disturbance of the ER quality control system (Jin et al. 2007; Hong et al. 2008, 2009). Consistent with our prediction, os9-1 partially suppresses the dwarf phenotype of bri1-9 (Fig. S5).
To show that the suppression of the phenotype is allele-specific we crossed os9-1 to bri1-6, which is another bri1 allele with a missense mutation (G644D) in the brassinosteroid binding domain (Noguchi et al. 1999) that does not result in ER retention of the mutated BRI1-6 protein, but very likely interferes with intra-molecular signal transduction of the brassinosteroid receptor (Kinoshita et al. 2005; Hong et al. 2008). As shown in Fig. 5d and Fig. S6 os9-1 fails to rescue the bri1-6 defect suggesting that OS9-mediated rescue of the bri1 growth defect is specific for the ER-retained forms of the mutated BRI1 receptor.
Interaction of BRI1-5 and BRI1-9 protein with OS9
Our data indicate that OS9 is involved in degradation of ER-retained BRI1 proteins. To investigate whether OS9 interacts with BRI1 proteins we expressed different BRI1 forms together with OS9-GFPglyc transiently in N. benthamiana leaves, purified the OS9-GFPglyc by Protein A affinity chromatography and performed immunoblots with anti-BRI1 antibodies to analyze the binding to OS9. In contrast to wild-type BRI1, BRI1-5 and BRI1-9 could be co-purified with OS9-GFPglyc (Fig. 6a). Like OS9, BRI1-5-GFP was found in the ER and displayed Endo H sensitive N-glycans, indicating that the interaction of OS9 and BRI1-5 takes place in the ER (Fig. S7).
OS9 interacts with the ERAD substrates BRI1-5 and BRI1-9. a BRI1 forms were transiently co-expressed with OS9-GFPglyc in N. benthamiana leaves. OS9-GFPglyc was purified and co-purified protein fractions were analyzed by SDS–PAGE and immunoblotting with anti-BRI1 and anti-OS9 antibodies. The asterisk indicates an unspecific band that was used as loading control. b As in a but expression was done in the presence of 20 μM kifunensine (kif)
In the presence of kifunensine association of OS9 with BRI1-5 was clearly reduced, being consistent with the idea that OS9 binding to ERAD substrates requires recognition of a distinct mannose residue generated by α-mannosidases (Fig. 6b). Interestingly the interaction of BRI1-9 was more resistant to kifunensine treatment highlighting differences between the two ERAD substrates that have also been described previously (Jin et al. 2007; Hong et al. 2008, 2009; Jin et al. 2009). To further investigate the role of the putative MRH domain for its function and interaction with ERAD clients we generated a mutant OS9 version where the arginine residue at position 201, which corresponds to R200 in YOS9, was mutated to alanine (OS9R201A mutant). For YOS9 it has been shown that this residue is critical for its lectin binding ability and subsequent ERAD of misfolded proteins (Bhamidipati et al. 2005; Szathmary et al. 2005; Quan et al. 2008). Consistent with our prediction the expression of OS9R201A-GFPglyc in os9-1 bri1-5 did not restore the dwarf phenotype of bri1-5 (Fig. S8).
OS9 expression is drastically reduced in the sel1l mutant
For mammalian cells it has been shown that SEL1L and OS-9 interact and it has been suggested that this interaction is dependent on the N-glycans present on SEL1L (Gauss et al. 2006; Christianson et al. 2008). Consequently, we asked the question whether A. thaliana SEL1L and OS9 can also associate. To this end we co-expressed OS9-GFPglyc and SEL1L containing a 3× HA-tag transiently in N. benthamiana and performed co-purification experiments. We observed that OS9 and SEL1L displayed a strong association. Importantly, this interaction was independent of kifunensine treatment (Fig. 7a) and was also found when the mutated OS9R201A-GFPglyc was used to co-purify SEL1L-HA (Fig. 7b).
OS9 interacts with SEL1L in a glycan-independent way. a HA-tagged A. thaliana SEL1L was co-expressed with OS9-GFPglyc in N. benthamiana. OS9-GFPglyc was purified and co-purified protein fractions were analyzed by immunoblotting with anti-HA antibodies. (+) and (−) indicates expression in the presence or absence of 20 μM kifunensine (kif). b As in a but the mutated OS9R201A-GFPglyc was used for purification of SEL1L. c Protein extracts from A. thaliana sel1l, wild-type (wt) and os9-1 were separated by SDS–PAGE and analyzed by immunoblotting with anti-OS9 antibodies. α-tubulin expression was used as a control. d As in c but wt with kif and alg12 are shown. alg12 is in Ws-4 background. OS9 is more abundant in alg12 than in Ws-4, which is very likely caused by the activation of the unfolded protein response in alg12 (Hong et al. 2009)
To see whether OS9 protein levels are altered in the absence of SEL1L (Liu et al. 2011; Su et al. 2011) we performed immunoblots with protein extracts from A. thaliana sel1l knockout seedlings (Fig. 7c). OS9 levels were considerably reduced in seedlings suggesting that SEL1L is required for OS9 stability or ER retention due to association in a complex. We investigated whether the interaction between OS9 and SEL1L is dependent on a defined glycan structure present on SEL1L, which is glycosylated in A. thaliana (Su et al. 2011). If so, the interaction should be abolished by kifunensine treatment or in glycosylation mutants like alg12 which produce aberrant N-glycan structures that block the ERAD pathway (Hong et al. 2009). OS9 protein levels were unchanged in wild-type seedlings in the presence of kifunensine as well as in alg12 plants (Fig. 7d). Together these data indicate that mannose trimming and the exposure of α1,6-mannosyl residues is not required for SEL1L-OS9 interaction.
The os9-1 mutant displays a salt stress phenotype
Recently it was shown that ERAD is necessary for plant salt tolerance as sel1l knockout seedlings displayed increased salt sensitivity (Liu et al. 2011). We subjected os9-1 plants to different salt treatments and analyzed the phenotype. Germination of seeds on MS medium with 120 mM NaCl showed that os9-1 seedlings are more sensitive towards NaCl than wild type (Fig. 8a). Moreover, os9-1 and sel1l seedlings were also more sensitive towards KCl (Fig. 8b) corroborating the finding that disruption of the ERAD pathway results in increased salt sensitivity. We also tested whether OS9 is important for the function of the heavily glycosylated receptor kinase EFR, which is required for the perception of the bacterial EF-Tu during pathogen infection (Zipfel et al. 2006). In previous studies it was shown that EFR is a client of the A. thaliana ER quality control system and mutations in diverse ER-chaperones and glycosylation enzymes affect its function (Li et al. 2009; Nekrasov et al. 2009; Saijo et al. 2009; Häweker et al. 2010). To test the effect of OS9 deficiency we performed a seedling growth inhibition assay (Fig. S9) in the presence of the bacterial peptide elf18. The os9-1 seedlings displayed a retarded growth phenotype similar to wild type indicating that OS9 is not required for EFR function.
os9-1 seedlings are sensitive to salt stress. a Wild-type (wt) and os9-1 seedlings were directly spread on ½× MS medium containing 1.5% sucrose supplemented with 120 mM NaCl and grown for 12 days. b Quantitative analysis of different seedling phenotypes grown for 12 days either on 120 mM NaCl or KCl. Percentages (vertical axis) represent smaller and yellow seedlings and are means ± SE from three independent repeats (more than 100 seedlings were counted per line and experiment, the total number of seedlings represents 100%). *P < 0.05 (paired Student’s t test)
It has also been shown that sel1l knockout plants are more sensitive to tunicamycin, which leads to underglycosylation of proteins and subsequent activation of the UPR, and to paraquat, an inducer of oxidative stress (Liu et al. 2011). However, os9-1 seedlings were less sensitive than sel1l mutants to both chemicals and appeared indistinguishable from wild-type seedlings (Fig. S10).
Discussion
The ERAD machinery identifies and degrades individual aberrant proteins and ensures together with the protein folding machinery that only properly folded and assembled proteins are released from the ER. The disposal of misfolded proteins is crucial to prevent the accumulation of defective proteins in the ER, which compromise its function leading to ER stress and eventually cell death. Deletion of ERAD components results in systemic ER stress and embryo lethality in mammals (Francisco et al. 2010). Despite its importance for protein homeostasis in the cell very little is known about the ERAD pathway in plants (Vitale and Boston 2008; Liu and Howell 2010a; Ceriotti 2011). The recent identification of the A. thaliana SEL1L/HRD3 and HRD1 homologs has highlighted the importance of ERAD components for cellular processes (Liu et al. 2011; Su et al. 2011). Here, we provide evidence that the so far uncharacterized A. thaliana OS9 protein functions in the glycoprotein ERAD pathway: (i) A. thaliana OS9 contains an MRH domain with homology to mammalian and yeast proteins involved in ERAD of glycosylated substrates. (ii) OS9 is an ER-located protein. (iii) OS9 containing the C-terminal domain of YOS9 can complement the Δyos9 yeast mutant. (iv) OS9 can suppress the bri1-5 and bri1-9 growth phenotypes and (v) interacts with these heavily glycosylated ERAD substrates as well as with the A. thaliana SEL1L protein. The association of OS9 with BRI1-5 is dependent on trimming of mannose residues and presumably requires an intact MRH domain. SEL1L, on the other hand, is involved in the degradation of glycosylated as well as non-glycosylated proteins presumably by association with misfolded protein domains exposed on ERAD substrates (Liu et al. 2011; Su et al. 2011).
The effect of OS9 deficiency on the stability of ERAD substrates remains to be shown. The suppression of the bri1-5 and bri1-9 dwarf phenotypes by OS9 deficiency indicates that BRI1-5 and BRI1-9 proteins can escape from the ER either due to accumulation in the absence of a specific degradation process or due to lack of OS9-specific ER-retention. We have not yet identified the oligosaccharide structure that constitutes, together with the misfolded polypeptide domain, the recognition signal for OS9. The presence of conserved residues in the MRH domain including the double tryptophan motif that has been suggested to confer specificity of human OS-9 for the terminal α1,6-mannose (Satoh et al. 2010) and the finding that ERAD is blocked in the Arabidopsis alg12 mutant that lacks this sugar residue (Hong et al. 2009) suggest that OS9 has a similar oligosaccharide binding specificity as found for yeast and mammalian homologs (Quan et al. 2008; Hosokawa et al. 2009). However, our data also indicate that there are fundamental variations between the different systems. OS9 can only restore ERAD of CPY* in Δyos9 yeast cells when it contains the C-terminal YOS9 domain, and the expression of YOS9 in Arabidopsis bri1-5 os9-1 mutants does not revert the bri1-5 os9-1 phenotype (data not shown). For YOS9 it has been shown that a region within amino acids 250–420 in its C-terminal domain is required for interaction with HRD3 (Gauss et al. 2006) and together YOS9 and HRD3 ensure that only terminally misfolded proteins are degraded. OS9 lacks this region, which can explain its non-function in yeast.
The molecular mechanisms of the glycan-dependent steps in the mammalian ERAD pathway are still unclear and two models have been proposed for the role of N-glycans and the ER-resident OS-9/XTP3-B lectins (Hebert et al. 2010; Hosokawa et al. 2010). In the more widely favoured model an oligosaccharide on a misfolded protein acts like in yeast as the degradation signal and is recognized by these lectins. The second model proposes that the OS-9/XTP3-B-SEL1L interaction is mediated by binding of the MRH domain to the N-glycans of SEL1L and association with ERAD substrates is independent of oligosaccharide binding (Christianson et al. 2008). In agreement with the former model, our data indicate that SEL1L-OS9 interaction in plants is glycan-independent, because interaction persisted when mannose trimming was abolished and the MRH domain was mutated. Since OS9 lacks any C-terminal YOS9 domain that is required for association with HRD3 our finding raises the so far unsolved question how OS9 associates with Arabidopsis SEL1L/HRD3 and the HRD-ligase complex that links substrate recognition with proteasomal degradation. Moreover, since OS9 protein levels are clearly reduced in the sel1l mutant we propose that OS9-SEL1L interaction plays also a role for the ER retention of OS9, which lacks any obvious ER-retention or retrieval signal.
Here, we show that OS9 interacts with both BRI1-5 and BRI1-9, but kifunensine treatment disturbed the association with BRI1-5 much more than with BRI1-9. The two distinct mutations affect very different regions of BRI1 (Hothorn et al. 2011; She et al. 2011) and genetic as well as biochemical studies have shown that the two mutated BRI1 forms have different requirements for ER quality control (Jin et al. 2007; Hong et al. 2008). Consequently our data argue that BRI1-5 and BRI1-9 represent different classes of ERAD substrates that require distinct components for degradation like it has also been proposed for mammalian ERAD substrates (Bernasconi et al. 2010). The interaction of BRI1-9 with OS9 might be more dependent on interaction with exposed protein segments and OS9 may—like YOS9—also bind and assist in degradation of certain non-glycosylated substrates (Bhamidipati et al. 2005; Jaenicke et al. 2011).
Which endogenous proteins are normally recognized by OS9 and selected for degradation through the ERAD pathway? The os9-1 mutant does not display any morphological phenotype and the overall protein levels do not appear drastically changed in the mutant. However, under abiotic stress conditions the os9-1 mutant displays a clear phenotype, which is in agreement with data for sel1l and the finding that salt stress leads to ER stress and presumably accumulation of misfolded proteins that have to be removed in order to prevent damage to the cells (Liu and Howell 2010a). Moreover, UPR triggered by tunicamycin or DTT treatment induces OS9 expression (Martínez and Chrispeels 2003; Liu and Howell 2010b), but interestingly, os9-1 seedlings display a similar response like wild type when treated with tunicamycin. Clearly, additional studies are needed to identify endogenous plant ERAD substrates and to dissect the important role of ERAD components under different stress conditions. We are still at the beginning of understanding this process for plants and our knowledge is restricted by the limited number of available plant ERAD substrates. However, the characterization of OS9 together with the recently identified ERAD components will help to elucidate the mechanism as well as the biological function of the pathway in the future.
References
Bernasconi R, Galli C, Calanca V, Nakajima T, Molinari M (2010) Stringent requirement for HRD1, SEL1L, and OS-9/XTP3-B for disposal of ERAD-LS substrates. J Cell Biol 188:223–235
Bhamidipati A, Denic V, Quan EM, Weissman JS (2005) Exploration of the topological requirements of ERAD identifies Yos9p as a lectin sensor of misfolded glycoproteins in the ER lumen. Mol Cell 19:741–751
Brandizzi F, Hanton S, DaSilva L, Boevink P, Evans D, Oparka K, Denecke J, Hawes C (2003) ER quality control can lead to retrograde transport from the ER lumen to the cytosol and the nucleoplasm in plants. Plant J 34:269–281
Ceriotti A (2011) Waste disposal in the endoplasmic reticulum, ROS production and plant salt stress response. Cell Res 21:555–557
Christianson JC, Shaler TA, Tyler RE, Kopito RR (2008) OS-9 and GRP94 deliver mutant α1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol 10:272–282
Clerc S, Hirsch C, Oggier D, Deprez P, Jakob C, Sommer T, Aebi M (2009) Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J Cell Biol 184:159–172
D‘Alessio C, Caramelo JJ, Parodi AJ (2010) UDP-GlC: glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin Cell Dev Biol 21:491–499
Elbein AD, Tropea JE, Mitchell M, Kaushal GP (1990) Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem 265:15599–15605
Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Natl Rev Mol Cell Biol 4:181–191
Farid A, Pabst M, Schoberer J, Altmann F, Glössl J, Strasser R (2011) Arabidopsis thaliana alpha1,2-glucosyltransferase (ALG10) is required for efficient N-glycosylation and leaf growth. Plant J 68:314–325
Francisco AB, Singh R, Li S, Vani AK, Yang L, Munroe RJ, Diaferia G, Cardano M, Biunno I, Qi L, Schimenti JC, Long Q (2010) Deficiency of suppressor enhancer Lin12 1 like (SEL1L) in mice leads to systemic endoplasmic reticulum stress and embryonic lethality. J Biol Chem 285:13694–13703
Gauss R, Jarosch E, Sommer T, Hirsch C (2006) A complex of Yos9p and the HRD ligase integrates endoplasmic reticulum quality control into the degradation machinery. Nat Cell Biol 8:849–854
Ghosh P, Dahms NM, Kornfeld S (2003) Mannose 6-phosphate receptors: new twists in the tale. Natl Rev Mol Cell Biol 4:202–212
Häweker H, Rips S, Koiwa H, Salomon S, Saijo Y, Chinchilla D, Robatzek S, von Schaewen A (2010) Pattern recognition receptors require N-glycosylation to mediate plant immunity. J Biol Chem 285:4629–4636
Hebert DN, Bernasconi R, Molinari M (2010) ERAD substrates: which way out? Semin Cell Dev Biol 21:526–532
Helenius A, Aebi M (2001) Intracellular functions of N-linked glycans. Science 291:2364–2369
Hong Z, Jin H, Tzfira T, Li J (2008) Multiple mechanism-mediated retention of a defective brassinosteroid receptor in the endoplasmic reticulum of Arabidopsis. Plant Cell 20:3418–3429
Hong Z, Jin H, Fitchette A, Xia Y, Monk A, Faye L, Li J (2009) Mutations of an α1,6 mannosyltransferase inhibit endoplasmic reticulum-associated degradation of defective brassinosteroid receptors in Arabidopsis. Plant Cell 21:3792–3802
Hosokawa N, Kamiya Y, Kamiya D, Kato K, Nagata K (2009) Human OS-9, a lectin required for glycoprotein endoplasmic reticulum-associated degradation, recognizes mannose-trimmed N-glycans. J Biol Chem 284:17061–17068
Hosokawa N, Kamiya Y, Kato K (2010) The role of MRH domain-containing lectins in ERAD. Glycobiology 20:651–660
Hothorn M, Belkhadir Y, Dreux M, Dabi T, Noel JP, Wilson IA, Chory J (2011) Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature 474:467–471
Ishiguro S, Watanabe Y, Ito N, Nonaka H, Takeda N, Sakai T, Kanaya H, Okada K (2002) SHEPHERD is the Arabidopsis GRP94 responsible for the formation of functional CLAVATA proteins. EMBO J 21:898–908
Jaenicke LA, Brendebach H, Selbach M, Hirsch C (2011) Yos9p assists in the degradation of certain non-glycosylated proteins from the endoplasmic reticulum. Mol Biol Cell 22:2937–2945
Jakob C, Burda P, Roth J, Aebi M (1998) Degradation of misfolded endoplasmic reticulum glycoproteins in Saccharomyces cerevisiae is determined by a specific oligosaccharide structure. J Cell Biol 142:1223–1233
Jin H, Yan Z, Nam K, Li J (2007) Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Mol Cell 26:821–830
Jin H, Hong Z, Su W, Li J (2009) A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proc Natl Acad Sci USA 106:13612–13617
Kinoshita T, Caño-Delgado A, Seto H, Hiranuma S, Fujioka S, Yoshida S, Chory J (2005) Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433:167–171
Knop M, Hauser N, Wolf D (1996) N-Glycosylation affects endoplasmic reticulum degradation of a mutated derivative of carboxypeptidase yscY in yeast. Yeast 12:1229–1238
Koiwa H, Li F, McCully M, Mendoza I, Koizumi N, Manabe Y, Nakagawa Y, Zhu J, Rus A, Pardo J, Bressan R, Hasegawa P (2003) The STT3a subunit isoform of the Arabidopsis oligosaccharyltransferase controls adaptive responses to salt/osmotic stress. Plant Cell 15:2273–2284
Lerouxel O, Mouille G, Andème-Onzighi C, Bruyant M, Séveno M, Loutelier-Bourhis C, Driouich A, Höfte H, Lerouge P (2005) Mutants in DEFECTIVE GLYCOSYLATION, an Arabidopsis homolog of an oligosaccharyltransferase complex subunit, show protein underglycosylation and defects in cell differentiation and growth. Plant J 42:455–468
Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90:929–938
Li J, Wen J, Lease K, Doke J, Tax F, Walker J (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110:213–222
Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chinchilla D, Zipfel C, Jones J (2009) Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci USA 106:15973–15978
Liebminger E, Hüttner S, Vavra U, Fischl R, Schoberer J, Grass J, Blaukopf C, Seifert G, Altmann F, Mach L, Strasser R (2009) Class I α-mannosidases are required for N-glycan processing and root development in Arabidopsis thaliana. Plant Cell 21:3850–3867
Liu JX, Howell SH (2010a) Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 22:2930–2942
Liu JX, Howell SH (2010b) bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 22:782–796
Liu L, Cui F, Li Q, Yin B, Zhang H, Liu B, Wu Y, Xia R, Tang S, Xie Q (2011) The endoplasmic reticulum-associated degradation is necessary for plant salt tolerance. Cell Res 21:957–969
Marshall R, Jolliffe N, Ceriotti A, Snowden C, Lord J, Frigerio L, Roberts L (2008) The role of CDC48 in the retro-translocation of non-ubiquitinated toxin substrates in plant cells. J Biol Chem 283:15869–15877
Martínez I, Chrispeels M (2003) Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 15:561–576
McCracken AA, Brodsky JL (1996) Assembly of ER-associated protein degradation in vitro: dependence on cytosol, calnexin, and ATP. J Cell Biol 132:291–298
Müller J, Piffanelli P, Devoto A, Miklis M, Elliott C, Ortmann B, Schulze-Lefert P, Panstruga R (2005) Conserved ERAD-like quality control of a plant polytopic membrane protein. Plant Cell 17:149–163
Munro S (2001) The MRH domain suggests a shared ancestry for the mannose 6-phosphate receptors and other N-glycan-recognising proteins. Curr Biol 11:R499–R501
Nekrasov V, Li J, Batoux M, Roux M, Chu Z, Lacombe S, Rougon A, Bittel P, Kiss-Papp M, Chinchilla D, van Esse H, Jorda L, Schwessinger B, Nicaise V, Thomma B, Molina A, Jones J, Zipfel C (2009) Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO J 28:3428–3438
Noguchi T, Fujioka S, Choe S, Takatsuto S, Yoshida S, Yuan H, Feldmann K, Tax F (1999) Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol 121:743–752
Quan E, Kamiya Y, Kamiya D, Denic V, Weibezahn J, Kato K, Weissman J (2008) Defining the glycan destruction signal for endoplasmic reticulum-associated degradation. Mol Cell 32:870–877
Quinn RP, Mahoney SJ, Wilkinson BM, Thornton DJ, Stirling CJ (2009) A novel role for Gtb1p in glucose trimming of N-linked glycans. Glycobiology 19:1408–1416
Roberts DL, Weix DJ, Dahms NM, Kim JJ (1998) Molecular basis of lysosomal enzyme recognition: three-dimensional structure of the cation-dependent mannose 6-phosphate receptor. Cell 93:639–648
Saijo Y, Tintor N, Lu X, Rauf P, Pajerowska-Mukhtar K, Häweker H, Dong X, Robatzek S, Schulze-Lefert P (2009) Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J 28:3439–3449
Satoh T, Chen Y, Hu D, Hanashima S, Yamamoto K, Yamaguchi Y (2010) Structural basis for oligosaccharide recognition of misfolded glycoproteins by OS-9 in ER-associated degradation. Mol Cell 40:905–916
Schoberer J, Vavra U, Stadlmann J, Hawes C, Mach L, Steinkellner H, Strasser R (2009) Arginine/lysine residues in the cytoplasmic tail promote ER export of plant glycosylation enzymes. Traffic 10:101–115
Schott A, Ravaud S, Keller S, Radzimanowski J, Viotti C, Hillmer S, Sinning I, Strahl S (2010) Arabidopsis stromal-derived Factor2 (SDF2) is a crucial target of the unfolded protein response in the endoplasmic reticulum. J Biol Chem 285:18113–18121
She J, Han Z, Kim TW, Wang J, Cheng W, Chang J, Shi S, Yang M, Wang ZY, Chai J (2011) Structural insight into brassinosteroid perception by BRI1. Nature 474:472–476
Soussilane P, Soussillane P, D‘Alessio C, Paccalet T, Fitchette A, Parodi A, Williamson R, Plasson C, Faye L, Gomord V (2009) N-glycan trimming by glucosidase II is essential for Arabidopsis development. Glycoconj J 26:597–607
Strasser R, Schoberer J, Jin C, Glössl J, Mach L, Steinkellner H (2006) Molecular cloning and characterization of Arabidopsis thaliana Golgi α-mannosidase II, a key enzyme in the formation of complex N-glycans in plants. Plant J 45:789–803
Strasser R, Bondili J, Vavra U, Schoberer J, Svoboda B, Glössl J, Léonard R, Stadlmann J, Altmann F, Steinkellner H, Mach L (2007) A unique beta1,3-galactosyltransferase is indispensable for the biosynthesis of N-glycans containing Lewis a structures in Arabidopsis thaliana. Plant Cell 19:2278–2292
Su W, Liu Y, Xia Y, Hong Z, Li J (2011) Conserved endoplasmic reticulum-associated degradation system to eliminate mutated receptor-like kinases in Arabidopsis. Proc Natl Acad Sci USA 108:870–875
Szathmary R, Bielmann R, Nita-Lazar M, Burda P, Jakob CA (2005) Yos9 protein is essential for degradation of misfolded glycoproteins and may function as lectin in ERAD. Mol Cell 19:765–775
Vembar S, Brodsky J (2008) One step at a time: endoplasmic reticulum-associated degradation. Natl Rev Mol Cell Biol 9:944–957
Vitale A, Boston R (2008) Endoplasmic reticulum quality control and the unfolded protein response: insights from plants. Traffic 9:1581–1588
Yamamoto M, Maruyama D, Endo T, Nishikawa S (2008) Arabidopsis thaliana has a set of J proteins in the endoplasmic reticulum that are conserved from yeast to animals and plants. Plant Cell Physiol 49:1547–1562
Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones J, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125:749–760
Acknowledgments
We thank Gerhard Adam for yeast strains and vector pADHfw, Lukas Mach for providing the anti-HA antibody and Karin Polacsek (all BOKU-Vienna) for N-glycan analysis. We thank Dieter H. Wolf (University of Stuttgart, Stuttgart, Germany) for the kind gift of CPY* expression vector pRS306-prc1-1, Frans E. Tax (University of Arizona, Tucson, AZ) for the kind gift of bri1-9 seeds, Cyril Zipfel (The Sainsbury Laboratories, Norwich, UK) for efr-1 seeds and elf18 peptide and Ikuko Hara-Nishimura (Department of Botany, Graduate School of Science, Kyoto University, Kyoto, Japan) for the kind gift of anti-TGG1 antibodies. This work was supported by a grant from Austrian Science Fund (FWF): P20817-B12.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hüttner, S., Veit, C., Schoberer, J. et al. Unraveling the function of Arabidopsis thaliana OS9 in the endoplasmic reticulum-associated degradation of glycoproteins. Plant Mol Biol 79, 21–33 (2012). https://doi.org/10.1007/s11103-012-9891-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-012-9891-4