Abstract
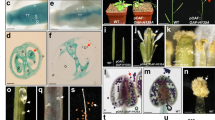
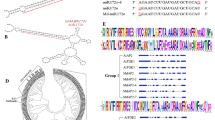
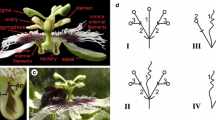
In Arabidopsis, the identity of perianth and reproductive organs are specified by antagonistic action of two floral homeotic genes, APETALA2 (AP2) and AGAMOUS (AG). AP2 is also negatively regulated by an evolutionary conserved interaction with a microRNA, miR172, and has additional roles in general plant development. A kiwifruit gene with high levels of homology to AP2 and AP2-like genes from other plant species was identified. The transcript was abundant in the kiwifruit flower, particularly petal, suggesting a role in floral organ identity. Splice variants were identified, all containing both AP2 domains, including a variant that potentially produces a shorter transcript without the miRNA172 targeting site. Increased AP2 transcript accumulation was detected in the aberrant flowers of the mutant ‘Pukekohe dwarf’ with multiple perianth whorls and extended petaloid features. In contrast to normal kiwifruit flowers, the aberrant flowers failed to accumulate miR172 in the developing whorls, although accumulation was detected at the base of the flower. An additional role during dormancy in kiwifruit was proposed based on AP2 transcript accumulation in axillary buds before and after budbreak.






Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15:2730–2741
Axtell MJ, Bartel DP (2005) Antiquity of microRNAs and their targets in land plants. Plant Cell Online 17:1658–1673
Bowman JL, Smyth DR, Meyerowitz EM (1989) Genes directing flower development in Arabidopsis. Plant Cell Online 1:37–52
Bowman JL, Smyth DR, Meyerowitz EM (1991) Genetic interactions among floral homeotic genes of Arabidopsis. Development 112:1–20
Brundell D (1975a) Flower development of the Chinese goosberry (Actinidia chinensis Planch.). II Development of the flowering bud. N Z J Bot 13:485–496
Brundell D (1975b) Flower development of the Chinese gooseberry (Actinidia chinensis Planch.). I. Development of the flower shoot. N Z J Bot 13:473–483
Brunner A, Yakovlev I, Strauss S (2004) Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol 4:14
Buhtz A, Springer F, Chappell L, Baulcombe DC, Kehr J (2008) Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J 53:739–749
Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303:2022–2025
Chuck G, Meeley RB, Hake S (1998) The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev 12:1145–1154
Chuck G, Meeley R, Irish E, Sakai H, Hake S (2007) The maize tasselseed4 microRNA controls sex determination and meristem cell fate by targeting tasselseed6/indeterminate spikelet1. Nat Genet 39:1517–1521
Coen ES, Meyerowitz EM (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353:31–37
Cox KH, DeLeon DV, Angerer LM, Angerer RC (1984) Detection of mRNAs in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol 101:485–502
Crowhurst R, Gleave A, MacRae E, Ampomah-Dwamena C, Atkinson R, Beuning L, Bulley S, Chagne D, Marsh K, Matich A, Montefiori M, Newcomb R, Schaffer R, Usadel B, Allan A, Boldingh H, Bowen J, Davy M, Eckloff R, Ferguson AR, Fraser L, Gera E, Hellens R, Janssen B, Klages K, Lo K, MacDiarmid R, Nain B, McNeilage M, Rassam M, Richardson A, Rikkerink E, Ross G, Schroder R, Snowden K, Souleyre E, Templeton M, Walton E, Wang D, Wang M, Wang Y, Wood M, Wu R, Yauk Y-K, Laing W (2008) Analysis of expressed sequence tags from Actinidia: applications of a cross species EST database for gene discovery in the areas of flavor, health, color and ripening. BMC Genomics 9:351
Ferguson AR (1984) Kiwifruit: a botanical review. Hortic Rev 6:1–64
Ferguson AR (1990) The genus Actinidia. In: Warrington IJ, Weston GC (ed) Kiwifruit: science and management, Ray Richards Publisher and New Zealand Society for Horticultural Science, Auckland, pp 15–35
Foster TM, Seleznyova AN, Barnett AM (2007) Independent control of organogenesis and shoot tip abortion are key factors to developmental plasticity in kiwifruit (Actinidia). Ann Bot 100:471–481
Gasic K, Hernandez A, Korban SS (2004) RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol Biol Rep 22:437a–437g
Grigorova B, Mara C, Hollender C, Sijacic P, Chen X, Liu Z (2011) LEUNIG and SEUSS co-repressors regulate miR172 expression in Arabidopsis flowers. Development 138:2451–2456
Gustafson-Brown C, Savidge B, Yanofsky MF (1994) Regulation of the Arabidopsis floral homeotic gene APETALA1. Cell 76:131–143
Huang HW, Ferguson AR (2007) Genetic resources of kiwifruit: domestication and breeding. Hortic Rev 33:1–121
Jack T, Brockman L, Meyerowitz E (1992) The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 68:683–697
Jofuku K, den Boer B, Van Montagu M, Okamuro J (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6:1211–1225
Jofuku K, Omidyar P, Gee Z, Okamuro J (2005) Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc Natl Acad Sci USA 102:3117–3122
Karlova R, Rosin FM, Busscher-Lange J, Parapunova V, Do PT, Fernie AR, Fraser PD, Baxter C, Angenent GC, de Maagd RA (2011) Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell 23:923–941
Kasai A, Kanehira A, Harada T (2010) miR172 can move long distances in Nicotiana benthamiana. Plant Sci J 4:1–6
Keck E, McSteen P, Carpenter R, Coen E (2003) Separation of genetic functions controlling organ identity in flowers. EMBO J 22:1058–1066
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Lee D-Y, Lee J, Moon S, Park SY, An G (2007) The rice heterochronic gene SUPERNUMERARY BRACT regulates the transition from spikelet meristem to floral meristem. Plant J 49:64–78
Litt A (2007) An evaluation of A-function: evidence from the APETALA1 and APETALA2 gene lineages. Int J Plant Sci 168:73–91
Llave C, Xie Z, Kasschau KD, Carrington JC (2002) Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297:2053–2056
Maes T, Van de Steene N, Zethof J, Karimi M, D’Hauw M, Mares G, Van Montagu M, Gerats T (2001) Petunia Ap2-like genes and their role in flower and seed development. Plant Cell 13:229–244
Martin A, Adam H, Díaz-Mendoza M, Żurczak M, González-Schain ND, Suárez-López P (2009) Graft-transmissible induction of potato tuberization by the microRNA miR172. Development 136:2873–2881
Nair SK, Wang N, Turuspekov Y, Pourkheirandish M, Sinsuwongwat S, Chen G, Sameri M, Tagiri A, Honda I, Watanabe Y, Kanamori H, Wicker T, Stein N, Nagamura Y, Matsumoto T, Komatsuda T (2010) Cleistogamous flowering in barley arises from the suppression of microRNA-guided HvAP2 mRNA cleavage. Proc Natl Acad Sci 107:490–495
Ohto MA, Fischer RL, Goldberg RB, Nakamura K, Harada JJ (2005) Control of seed mass by APETALA2. Proc Natl Acad Sci USA 102:3123–3128
Ohto MA, Floyd SK, Fischer RL, Goldberg RB, Harada JJ (2009) Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sex Plant Reprod 22:277–289
Okamuro JK, Caster B, Villarroel R, Van Montagu M, Jofuku KD (1997) The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc Natl Acad Sci 94:7076–7081
Park W, Li J, Song R, Messing J, Chen X (2002) CARPEL FACTORY, a dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12:1484–1495
Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405:200–203
Polito VS, Grant JA (1984) Initiation and development of pistillate flowers in Actinidia chinensis. Sci Hortic 22:365–371
Ripoll JJ, Roeder AH, Ditta GS, Yanofsky MF (2011) A novel role for the floral homeotic gene APETALA2 during Arabidopsis fruit development. Development 138:5167–5176
Snowball A (1997) Seasonal cycle of shoot development in selected Actinidia species. N Z J Crop Hortic Sci 25:221–231
Steel JH, Gordon L, Polak JM (1998) Principles and applications of complementary RNA probes. In: Polak J, JOD McGee (ed) In situ hybridization: principles and practice, 2nd edn. Oxford University Press, Oxford, NY, pp 49–69
Theissen G, Saedler H (2001) Plant biology. Floral quartets. Nature 409:469–471
Valoczi A, Varallyay E, Kauppinen S, Burgyan J, Havelda Z (2006) Spatio-temporal accumulation of microRNAs is highly coordinated in developing plant tissues. Plant J 47:140–151
Varkonyi-Gasic E, Hellens RP (2011) Quantitative stem-loop RT-PCR for detection of microRNAS. In: Kodama H, Komamine A (eds) RNAi and plant gene function analysis methods in molecular biology, vol 744. Springer, New York, NY, pp 145–157
Varkonyi-Gasic E, Gould N, Sandanayaka M, Sutherland P, MacDiarmid R (2010) Characterisation of microRNAs from apple (Malus domestica ‘Royal Gala’) vascular tissue and phloem sap. BMC Plant Biol 10:159
Varkonyi-Gasic E, Moss S, Voogd C, Wu R, Lough R, Wang Y-Y, Hellens R (2011) Identification and characterization of flowering genes in kiwifruit: sequence conservation and role in kiwifruit flower development. BMC Plant Biol 11:72
Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, Pruss D, Pindo M, FitzGerald LM, Vezzulli S, Reid J, Malacarne G, Iliev D, Coppola G, Wardell B, Micheletti D, Macalma T, Facci M, Mitchell JT, Perazzolli M, Eldredge G, Gatto P, Oyzerski R, Moretto M, Gutin N, Stefanini M, Chen Y, Segala C, Davenport C, Demattè L, Mraz A, Battilana J, Stormo K, Costa F, Tao Q, Si-Ammour A, Harkins T, Lackey A, Perbost C, Taillon B, Stella A, Solovyev V, Fawcett JA, Sterck L, Vandepoele K, Grando SM, Toppo S, Moser C, Lanchbury J, Bogden R, Skolnick M, Sgaramella V, Bhatnagar SK, Fontana P, Gutin A, Van de Peer Y, Salamini F, Viola R (2007) A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2:e1326
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Salvi S, Pindo M, Baldi P, Castelletti S, Cavaiuolo M, Coppola G, Costa F, Cova V, Dal Ri A, Goremykin V, Komjanc M, Longhi S, Magnago P, Malacarne G, Malnoy M, Micheletti D, Moretto M, Perazzolli M, Si-Ammour A, Vezzulli S, Zini E, Eldredge G, Fitzgerald LM, Gutin N, Lanchbury J, Macalma T, Mitchell JT, Reid J, Wardell B, Kodira C, Chen Z, Desany B, Niazi F, Palmer M, Koepke T, Jiwan D, Schaeffer S, Krishnan V, Wu C, Chu VT, King ST, Vick J, Tao Q, Mraz A, Stormo A, Stormo K, Bogden R, Ederle D, Stella A, Vecchietti A, Kater MM, Masiero S, Lasserre P, Lespinasse Y, Allan AC, Bus V, Chagne D, Crowhurst RN, Gleave AP, Lavezzo E, Fawcett JA, Proost S, Rouze P, Sterck L, Toppo S, Lazzari B, Hellens RP, Durel C-E, Gutin A, Bumgarner RE, Gardiner SE, Skolnick M, Egholm M, Van de Peer Y, Salamini F, Viola R (2010) The genome of the domesticated apple (Malus x domestica Borkh.). Nat Genet 42:833–839
Walton EF, Fowke PJ, Weis K, McLeay PL (1997) Shoot axillary bud morphogenesis in kiwifruit (Actinidia deliciosa). Ann Bot 80:13–21
Walton EF, Podivinsky E, Wu RM (2001) Bimodal patterns of floral gene expression over the two seasons that kiwifruit flowers develop. Physiol Plant 111:396–404
Walton EF, Wu RM, Richardson AC, Davy M, Hellens RP, Thodey K, Janssen BJ, Gleave AP, Rae GM, Wood M, Schaffer RJ (2009) A rapid transcriptional activation is induced by the dormancy-breaking chemical hydrogen cyanamide in kiwifruit (Actinidia deliciosa) buds. J Exp Bot 60:3835–3848
Weigel D, Meyerowitz E (1994) The ABCs of floral homeotic genes. Cell 78:203–209
Wollmann H, Mica E, Todesco M, Long JA, Weigel D (2010) On reconciling the interactions between APETALA2, miR172 and AGAMOUS with the ABC model of flower development. Development 137:3633–3642
Wurschum T, Gross-Hardt R, Laux T (2006) APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 18:295–307
Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM (1990) The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346:35–39
Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, Wollmann H, Chen X, Schmid M (2010) Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22:2156–2170
Yoo BC, Kragler F, Varkonyi-Gasic E, Haywood V, Archer-Evans S, Lee YM, Lough TJ, Lucas WJ (2004) A systemic small RNA signaling system in plants. Plant Cell 16:1979–2000
Zhao L, Kim Y, Dinh TT, Chen X (2007) miR172 regulates stem cell fate and defines the inner boundary of APETALA3 and PISTILLATA expression domain in Arabidopsis floral meristems. Plant J 51:840–849
Zhu Q-H, Upadhyaya N, Gubler F, Helliwell C (2009) Over-expression of miR172 causes loss of spikelet determinacy and floral organ abnormalities in rice (Oryza sativa). BMC Plant Biol 9:149
Acknowledgements
We would like to thank Mark McNeilage for help with the ‘Pukekohe dwarf’ somatic mutant, Tim Holmes for photography, and Anne Gunson for critical reading of the manuscript. This work was funded by the New Zealand Foundation for Research Science and Technology grant C10 × 0816 MeriNET.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Varkonyi-Gasic, E., Lough, R.H., Moss, S.M.A. et al. Kiwifruit floral gene APETALA2 is alternatively spliced and accumulates in aberrant indeterminate flowers in the absence of miR172. Plant Mol Biol 78, 417–429 (2012). https://doi.org/10.1007/s11103-012-9877-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-012-9877-2