Abstract
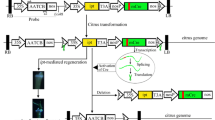
The COOH terminal of pthA encoding three nuclear localizing signals (NLS) was amplified by polymerase chain reaction (PCR) from the plasmid of Xanthomonas axonopodis pv. citri, the pathogen of citrus canker disease. Then the sense and antisense strands of the nls were cloned into pBI121 vector. pthA-nls driven by the CaMV35 s promoter was transferred into sweet orange via Agrobacterium -mediated transformation. Successful integration was confirmed by PCR and Southern blotting, and 12 sense-nls (nls +) and 9 antisense-nls (nls −) transgenic clones were obtained. The expression of nls fragment was analyzed by RT–PCR, Real time q-PCR and Western blotting, in which the specific NLS protein was detected only in nls + transgenic clones. In an in vitro assay, when pin-puncture inoculation was performed with 2.5 × 107 cfu/ml of bacterial solution, the nls + transgenic clones showed no typical lesion development, while typical symptoms were observed in the wild types and the nls − transgenic clones. In vivo assay results indicated that the nls + transgenic clones showed less disease incidence, in comparison with the wild types and the nls − transgenic clones, when pin-puncture inoculation was performed with 104–105 cfu/ml. The minimum disease incidence was 23.3% for ‘Sucarri’ sweet orange and 33.3% for ‘Bingtang’ sweet orange. When 104–107 cfu/ml of pathogen was spray inoculated, the nls + transgenic clones did not show any symptom, and even the concentration raised to 109 cfu/ml, the disease incidence was 20–80%, while the wild types and the nls − transgenic clones had 100% disease development with whatever concentration of inoculum. Two transgenic clones were confirmed to be resistant to citrus canker disease in the repeated inoculation. The results suggested that the transformation of nls sense strands may offer an effective way to acquire resistance to citrus canker disease.








Similar content being viewed by others
Abbreviations
- nls + :
-
Sense-nls strand
- nls − :
-
Antisense-nls strand
- HR:
-
Hypersensitive response
- NLSs:
-
Three nuclear localizing signals
- BAP:
-
Benzylaminopurine
References
Barbosa-Mendes JM, Mourao Filho FAA, Filho AB, Harakava R, Beer SV, Mendes BMJ (2009) Genetic transformation of Citrus sinensis cv. Hamlin with hrpN gene from Erwinia amylovora and evaluation of the transgenic lines for resistance to citrus canker. Sci Hortic 122:109–115
Baulcombe DC (1996) Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell 8:1833–1844
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen SC, Zhang JR, Huang ZR, Gao F, Chen FZ, Long YQ (1997) Studies on Agrobacterium-mediated antibacterial peptide D gene transfer in citrus. Sci Agric Sin 30:7–13 (in Chinese, abstract in English)
Civerolo EL (1984) Bacterial canker disease of citrus. J Rio Gd Val Hortic Soc 37:127–146
Dangl JL, Dietrich RA, Richberg MH (1996) Death don’t have no mercy: cell death program in plant-microbe interactions. Plant Cell 8:1793–1807
Das AK (2003) Citrus canker—a review. J Appl Hortic 5:52–60
Deng ZN, Gentile A, Nicolosi E, Domina VA, Tribulato E (1995) Identification of in vivo and in vitro lemon mutants by RAPD markers. J Hortic Sci 70:117–125
Distefano G, La Malfa S, Vitale A, Lorito M, Deng ZN, Gentile A (2008) Defence-related gene expression in transgenic lemon plants producing an antimicrobial Trichoderma harzianum endochitinase during fungal infection. Transgenic Res 17:873–879
Gabriel DW (1999) Why do pathogens carry avirulence genes? Physiol Mol Plant Pathol 55:205–214
Gabriel DW, Yuan Q, Yang Y, Chakrabarty PK (1996) Role of nuclear localizing signal sequences in three disease phenotypes determined by the Xanthomonas avr/pth gene family. Biology of plant-microbe interactions. In: Stacey G, Mullin B, Gresshoff PM (eds) Proceedings of international symposium on molecular plant-microbe interactions, 8th International Society for Molecular-Plant Microbe Interactions, St. Paul, MN, USA, pp 197–202
Gentile A, Deng ZN, La Malfa S, Domina F, Vitale A, Polizzi G, Lorito M, Tribulato E (2006) Enhanced resistance to Phoma tracheiphila and Botrytis cinerea in transgenic lemon plants expressing a Trichoderma harzianum chitinase gene. Plant Breed 125:1–6
Ghorbel R, Juarez J, Navarro L, Peña L (1999) Green fluorescent protein as a screenable marker to increase the efficiency of generating transgenic woody fruit plants. Theor Appl Genet 99:350–358
Gottwald TR, Graham JH, Schubert TS (2002) Citrus canker: the pathogen and its impact. On line. Plant Health Prog
Graham JH, Gottwald TR, Cubero J, Achor DS (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol Plant Pathol 1:1–15
Hu CH, Xu L, Ma XF, Long GY, Liu KY, Deng ZN (2008) Preparation of antiserum of the recombinant pthA-NLS and its inhibition effect on citrus canker disease. Acta Hortic Sin 35:811–818 (in Chinese, abstract in English)
Karen-Beth GS, Herman BS, Andrew OJ (1993) Control of plant virus diseases by pathogen-derived resistance in transgenic plants. Plant Physiol 102:7–12
Koizumi M, Kuhara S (1982) Evaluation of citrus plants for resistances to bacterial canker disease in relation to lesion extension. Bull Tree Fruit Res Stn Jpn D 4:73–92
Lahaye T, Bonas U (2001) Molecular secrets of bacterial type III effector proteins. Trends Plant Sci 6:479–485
Li N, Qin L, Yan JW, Chen P, Deng ZN (2010) Extraction efficiently of citrus leaf protein and Western Blot of the Citrus Canker Protein PthA. J Hunan Agric Univ (Nat Sci) 3:30–33 (in Chinese, abstract in English)
Luo SN, Zhong XH, Deng ZN, Huang RF (2008) Studies on Introducing TERF1 Gene into Sweet Orange. Acta Agric Univ Jiangxiensis 30:623–627 (in Chinese, abstract in English)
Ma XF, Han J, Hu CH, Li F, Dai SM, Deng ZN (2008) Molecular detection and characterization of citrus viriods. Sci Agric Sin 41:2670–2677
Mackintosh CA, Lewis J, Radmer LE, Shin S, Heinen SJ, Smith LA, Wyckoff MN, Dill-Macky R, Evans CK, Kravchenko S, Baldridge GD, Zeyen RJ, Muehlbauer GJ (2007) Overexpression of defense response genes in transgenic wheat enhances resistance to Fusarium head blight. Plant Cell Rep 26:479–488
Mccollum TG, Bowman KD, Gottwald TR (2006) Screening citrus germplasm for resistance to xanthomonas axonopodis pv. citri. In: Proceedings of the International Citrus Canker and Huanglongbing Research Workshop 2005, Florida Citrus Mutual, Orlando P18, p 3
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Peña L, Pérez RM, Cervera M, Juarez JA, Navarro L (2004) Early events in Agrobacterium-mediated genetic transformation of citrus explants. Ann Bot 94:67–74
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schubert TS, Rizvi SA, Sun XA, Gottwald TR, Graham JH, Dixon WN (2001) Meeting the challenge of eradicating citrus canker in Florida—again. Plant Dis 85:340–356
Swarup S, De Feyter R, Brlansky RH, Gabriel DW (1991) A pathogenicity locus from Xanthomonas citri enables strains from several pathovars of X. campestris to elicit canker like lesions on citrus. Phytopathology 81:802–809
Swarup S, Yang Y, Kingsley MT, Gabriel DW (1992) An Xanthomonas citri pathogenicity gene, pthA, pleiotropically encodes gratuitous avirulence on nonhosts. Mol Plant Microbe Interact 5:204–213
Vernière C, Hartung JS, Pruvost OP, Civerolo EL, Alvarez AM, Maestri P, Luisetti J (1998) Characterization of phenotypically distinct strains of Xanthomonas axonopodis pv. citri from southwest Asia. Eur J Plant Pathol 104:477–487
White PR (1951) Nutritional requirements of isolated plant tissues and organs. Ann Rev Plant Physiol 2:231
Xiang X (2006) Progresses on molecular breeding for citrus disease resistance. Mol Plant Breed 2:262–268 (in Chinese, abstract in English)
Xie YM, Deng ZN, Guo C, Jiao L, Hu CH, Xiong XY (2005) Preliminary studies on Agrobacterium-mediated transformation of sweet orange. J Hunan Agric Univ (Nat Sci) 31:627–630 655 (in Chinese, abstract in English)
Yang Y, Gabriel DW (1995a) Xanthomonas avirulence/pathogenicity gene family encodes functional plant nuclear targeting signals. Mol Plant Microbe Interact 8:627–631
Yang Y, Gabriel DW (1995b) Intragenic recombination of a single plant pathogen gene provides a mechanism for the evolution of new host specificities. J Bacteriol 177:4963–4968
Yang Y, Defeyter R, Gabriel DW (1994) Host-specific symptoms and increased release of Xanthomonas citri and Xanthomonas campestris pv. malvacearum from leaves are determined by the 102-bp tandem repeats of PthA and Avrb6, respectively. Mol Plant Microbe Interact 7:345–355
Zheng QF, Hu GB, Chen DC, Huang ZR, Lin SQ (2006) Evaluation on the transgenic plantlets of Citrus grandis cv. Shatian Pummelo with antibacterial peptide D gene for resistance to Xanthomonas axonopodis pv. citri. In: Seventh youth symposium of Chinese Society for Horticultural Science, pp 658–662 (in Chinese, abstract in English)
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30871702).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Li Yang and Chunhua Hu contributed equally to this article.
Rights and permissions
About this article
Cite this article
Yang, L., Hu, C., Li, N. et al. Transformation of sweet orange [Citrus sinensis (L.) Osbeck] with pthA-nls for acquiring resistance to citrus canker disease. Plant Mol Biol 75, 11–23 (2011). https://doi.org/10.1007/s11103-010-9699-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9699-z