Abstract
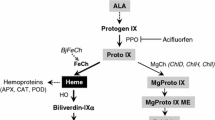
We generated transgenic rice plants (Oryza sativa cv. Dongjin) over-expressing human protoporphyrinogen IX oxidase (PPO) with the aim to increase mitochondrial PPO activity and confer herbicide resistance (Lee et al., Pestic Biochem Physiol 80:65–74, 2004). The transgenic plants showed during further leaf development the formation of severe necrotic spots and growth retardation. Several experiments were performed to examine the reasons for the formation of necrotic leaf lesions. Human PPO is normally located in mitochondria. An in vitro organellar import experiment revealed translocation of human PPO into pea chloroplasts, but not into mitochondria. Using a specific antibody raised against human PPO confirmed its plastidic localisation. The heme and chlorophyll contents were lower in necrotic leaves than wild-type leaves. Interestingly, mature and necrotic leaves of 12-week-old transgenic plants contained up to 14- and 24-fold more protoporphyrin IX, respectively, than mature wild-type leaves. Enhanced levels of Mg-Protoporphyrin IX, Mg-Protoporphyrin IX monomethyl ester and protochlorophyllide were concurrently observed in transgenic plants relative to wild type. Accumulated porphyrins and Mg-porphyrins likely act as photosensitizers and cause high formation of the reactive oxygen species. These high levels of tetrapyrrole intermediates correlated with increased rates of 5-aminolevulinic acid synthesis in transgenic plants. Tetrapyrrole-induced photooxidation was confirmed by increased lipid peroxidation and subsequent cell death. The transgenic phenotype is the consequence of a highly modified tetrapyrrole metabolism due to additional expression of human PPO. A possible regulatory role of PPO in graminaceous seedlings is discussed.





Similar content being viewed by others
References
Alawady AE, Grimm B (2005) Tobacco Mg-protoporphyrin IX methyltransferase is involved in inverse activation of Mg-porphyrin and protoheme synthesis. Plant J 41:282–290
Bruce BD, Perry S, Froelich J, Keegstra K (1994) In vitro import of proteins into chloroplasts. In: Gelvin SB, Schilferoot RA (eds) Plant molecular biology manual, 2nd edn. Kluwer Academic Publishers, The Netherlands, pp J1–J15
Buege TA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Camadro J-M, Thome F, Brouillet N, Labbe P (1994) Purification and properties of protoporphyrinogen oxidase from the yeast Saccharomyces cerevisiae. Mitochondrial location and evidence for a precursor form of the protein. J Biol Chem 269:32085–32091
Choi KW, Han O, Lee HJ, Yun YC, Moon YH, Kim M, Kuk YI, Han SU, Guh JO (1998) Generation of resistance to the diphenyl ether herbicide, oxyfluorfen, via expression of the Bacillus subtilis protoporphyrinogen oxidase gene in transgenic tobacco plants. Biosci Biotechnol Biochem 62:558–560
Cornah JE, Roper JM, Singh DP, Smith AG (2002) Measurement of ferrochelatase activity using a novel assay suggests that plastids are the major site of haem biosynthesis in both photosynthetic and nonphotosynthetic cells of pea (Pisum sativum L.). Biochem J 362:423–432
Dailey TA, Dailey HA, Meissner P, Prasad AR (1995) Cloning, sequence, and expression of mouse protoporphyrinogen oxidase. Arch Biochem Biophy 324:379–384
Day DA, Neuberger M, Douce R (1985) Biochemical characterization of chlorophyll-free mitochondria from pea leaves. Aus J Plant Physiol 12:219–228
Dolphin D (1994) Photomedicine and photodynamic therapy. Can J Chem 72:1005–1013
Falk K, Behal RH, Xiang C, Oliver DJ (1998) Metabolic bypass of the tricarboxylic acid cycle during lipid mobilization in germinating oilseeds. Plant Physiol 117:473–481
Ferreira GC, Andrew TL, Kair SW, Dailey HA (1988) Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J Biol Chem 263:3835–3839
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717
Girotti AW, Kriska T (2004) Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxid Redox Sign 6:301–310
Goslings D, Meskauskiene R, Kim C, Lee KP, Nater M, Apel K (2004) Concurrent interactions of heme and FLU with Glu tRNA reductase (HEMA1), the target of metaboic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J 40:957–967
Ha SB, Lee SB, Lee Y, Yang K, Lee N, Jang SM, Chung JS, Jung S, Kim YS, Wi SG, Back K (2004) The plastidic Arabidopsis protoporphyrinogen IX oxidase gene, with or without the transit sequence, confers resistance to the diphenyl ether herbicide in rice. Plant Cell Environ 27:79–88
Hörtensteiner S (2006) Chlorophyll degradation during senescence. Annu Rev Plant Biol 57:55–77
Ichinose K, Che F-S, Kimura Y, Matsunobu A, Sato F, Yoshida S (1995) Selection and characterization of protoporphyrinogen oxidase inhibiting herbicide (S23142) resistant photomixotrophic cultured cells of Nicotiana tabacum. J Plant Physiol 146:693–698
Jacobs JM, Jacobs NJ, Sherman TD, Duke SO (1991) Effects of diphenyl ether herbicides on oxidation of protoporphyrinogen to protoporphyrin in organellar and plasma membrane enriched fractions of barley. Plant Physiol 97:197–203
Joshi PC, Pathak MA (1984) The role of active oxygen (1O2 and O −2 ) induced by crude coal tar and its ingredients used in photochemotherapy of skin diseases. J Invest Dermatol 82:67–73
Jung S, Chung JS, Jang SM, Guh JO, Lee HJ, Chon S-U, Kim K-M, Ha SB, Back K (2003) Either soluble or plastidic expression of recombinant protoporphyrinogen oxidase modulates tetrapyrrole biosynthesis and photosynthetic efficiency in transgenic rice. Biosci Biotechnol Biochem 67:1472–1478
Jung S, Lee Y, Yang K, Lee SB, Jang SM, Ha SB, Back K (2004) Dual targeting of Myxococcus xanthus protoporphyrinogen oxidase into chloroplasts and mitochondria and high-level oxyfluorfen resistance. Plant Cell Environ 27:1436–1446
Keetman U, Mock H-P, Grimm B (2002) Kinetics of antioxidative defense responses to photosensitisation in porphyrin-accumulating tobacco plants. Plant Physiol Biochem 40:697–707
Lee HJ, Duke SO (1994) Protoporphyrinogen IX-oxidizing activities involved in the mode of action of peroxidizing herbicides. J Agric Food Chem 42:2610–2618
Lee HJ, Duke MV, Duke SO (1993) Cellular localization of protoporphyrinogen-oxidizing activities of etiolated barley (Hordeum vulgare L.) leaves. Plant Physiol 102:881–889
Lee HJ, Lee SB, Chung JS, Han SU, Han O, Guh JO, Jeon JS, An G, Back K (2000) Transgenic rice plants expressing a Bacillus subtilis protoporphyrinogen oxidase gene are resistant to diphenyl ether herbicide oxyfluorfen. Plant Cell Physiol 41:743–749
Lee Y, Jung S, Back K (2004) Expression of human protoporphyrinogen oxidase in transgenic rice induces both a photodynamic response and oxyfluorfen resistance. Pestic Biochem Physiol 80:65–74
Lermontova I, Grimm B (2000) Overexpression of plastidic protoporphyrinogen IX oxidase leads to resistance to the diphenyl-ether herbicide acifluorfen. Plant Physiol 122:75–83
Lermontova I, Kruse E, Mock H, Grimm B (1997) Cloning and characterization of a plastidal and a mitochondrial isoform of tobacco protoporphyrinogen IX oxidase. Proc Natl Acad Sci USA 94:8895–8900
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lis R, Atteia A, Nogaj LA, Beale SI (2005) Subcellular localization and light-regulated expression of protoporphyrinogen IX oxidase and ferrochelatase in Chlamydomonas reinhardtii. Plant Physiol 139:1946–1958
Mach JM, Castillo AR, Hoogstraten R, Greenberg JT (2001) The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA 98:771–776
Meskauskiene R, Apel K (2002) Interaction of FLU, a negative regulator of tetrapyrrole biosynthesis, with the glutamyl-tRNA reductase requires the tetratricopeptide repeat domain of FLU. FEBS Lett 532:27–30
Nishimura K, Taketani S, Inokuchi H (1995) Cloning of a human cDNA for protoporphyrinogen oxidase by complementation in vivo of a hemG mutant of Escherichia coli. J Biol Chem 270:8076–8080
Papenbrock J, Grimm B (2001) Regulatory network of tetrapyrrole biosynthesis—studies of intracellular signaling involved in metabolic and developmental control of plastids. Planta 213:667–681
Papenbrock J, Mock HP, Kruse E, Grimm B (1999) Expression studies in tetrapyrrole biosynthesis–Inverse maxima of magnesium chelatase and ferrochelatase. Planta 208:264–273
Rodermel S (1999) Subunit control of rubisco biosynthesis––a relic of an endosymbiotic past? Photosynth Res 59:105–123
Rudhe C, Chew O, Whelan J, Glaser E (2002) A novel in vitro system for simultaneous import of precursor proteins into mitochondria and chloroplasts. Plant J 30:213–220
Slater TF (1984) Overview of methods used for detecting lipid peroxidation. Method Enzymol 105:283–293
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J 11:1187–1194
Tsuji H, Tsutsumi N, Sasaki T, Hirai A, Nakazono M (2003) Organ-specific expressions and chromosomal locations of two mitochondrial aldehyde dehydrogenase genes from rice (Oryza sativa L.), ALDH2a and ALDH2b. Gene 305:195–204
Valenzeno D (1987) Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem Photobiol 46:146–160
Von und zu Fraunberg M, Nyrönen T, Kauppinen R (2003) Mitochondrial targeting of normal and mutant protoporphyrinogen oxidase. J Biol Chem 278:13376–13381
Watanabe N, Che F-S, Iwano M, Takayama S, Yoshida S, Isogai A (2001) Dual targeting of spinach protoporphyrinogen oxidase II to mitochondria and chloroplasts by alternative use of two in-frame initiation codons. J Biol Chem 276:20474–20481
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation to the Agricultural Plant Stress Research Center (R11-2001-09203001-0) of Chonnam National University and a fellowship (NaFög-Promotionsförderung des Landes Berlin) to H.J.L.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, S., Lee, HJ., Lee, Y. et al. Toxic tetrapyrrole accumulation in protoporphyrinogen IX oxidase-overexpressing transgenic rice plants. Plant Mol Biol 67, 535–546 (2008). https://doi.org/10.1007/s11103-008-9338-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-008-9338-0