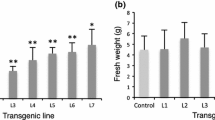
Abstract
Rice production is known to be severely affected by virus transmitting rice pests, brown planthopper (BPH) and green leafhopper (GLH) of the order hemiptera, feeding by phloem abstraction. ASAL, a novel lectin from leaves of garlic (Allium sativum) was previously demonstrated to be toxic towards hemipteran pests when administered in artificial diet as well as in ASAL expressing transgenic plants. In this report ASAL was targeted under the control of phloem-specific Agrobacterium rolC and rice sucrose synthase-1 (RSs1) promoters at the insect feeding site into popular rice cultivar, susceptible to hemipteran pests. PCR, Southern blot and C-PRINS analyses of transgenic plants have confirmed stable T-DNA integration and the transgenes were co-segregated among self-fertilized progenies. The T0 and T1 plants, harbouring single copy of intact T-DNA expression cassette, exhibit stable expression of ASAL in northern and western blot analyses. ELISA showed that the level of expressed ASAL was as high as 1.01% of total soluble protein. Immunohistofluorescence localization of ASAL depicted the expected expression patterns regulated by each promoter type. In-planta bioassay studies revealed that transgenic ASAL adversely affect survival, growth and population of BPH and GLH. GLH resistant T1 plants were further evaluated for the incidence of tungro disease, caused by co-infection of GLH vectored Rice tungro bacilliform virus (RTBV) and Rice tungro spherical virus (RTSV), which appeared to be dramatically reduced. The result presented here is the first report of such GLH mediated resistance to infection by RTBV/RTSV in ASAL expressing transgenic rice plant.
Similar content being viewed by others
Abbreviations
- ASAL:
-
Allium sativum agglutinin from leaf
- BAP:
-
benzylamino purine
- BPH:
-
brown planthopper
- BSA:
-
bovine serum albumin
- cv.:
-
cultivar
- C-PRINS:
-
cycling-primed in situ labelling
- dpi:
-
days post inoculation
- ELISA:
-
enzyme linked immunosorbent assay
- FITC:
-
fluorescein isothiocyanate
- GLH:
-
green leafhopper
- Hyg:
-
hygromycin
- MS:
-
Murashige and Skoog
- NAA:
-
α-napthaline acetic acid
- PBS:
-
phosphate buffered saline
- RSs1:
-
rice sucrose synthase-1 promoter
- RTBV:
-
Rice tungro bacilliform virus
- RTSV:
-
Rice tungro spherical virus
- SDS-PAGE:
-
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
References
Abbo S, Dunford RP, Miller TE, Reader SM, King IP (1993) Primer-mediated in situ detection of the B-hordein gene cluster on barley chromosome1H. Proc Natl Acad Sci USA 90:11821–11824
Bandyopadhyay S, Roy A, Das S (2001) Binding of garlic (Allium sativum) leaf lectin to the gut receptors of homopteran pests is correlated to its insecticidal activity. Plant Sci 161:1025–1033
Banerjee S, Hess D, Majumder P, Roy D, Das S (2004) The interactions of Allium sativum leaf agglutinin with a chaperonin group of unique receptor protein isolated from a bacterial endosymbiont of the mustard aphid. J Biol Chem 279:23782–23789
Barre A, Van Damme EJM, Peumaus WJ, Rouge P (1996) Structure-function relationship of monocot mannose-binding lectins. Plant Physiol 112:1531–1540
Bennet J (2001) Summing-up: cutting-edge science for rice improvement-breakthroughs and beneficiaries. In: Goode J, Chadwick D (Eds.), Rice biotechnology: improving yield, stress tolerance and grain quality, Novartis Foundation/John Wiley, UK, pp. 242–251
Bradford MM (1976) A rapid and sensitive method for the quantitation of proteins using the principle of protein-dye binding. Anal Biochem 72:248–254
Chang T, Chen L, Chen S, Cai H, Liu X, Xiao G, Zhu Z (2003) Transformation of tobacco with genes encoding Helianthus tuberosus agglutinin (HTA) confers resistance to peach-potato aphid (Myzus persicae). Transgenic Res 12:607–614
Dahal G, Hibino H, Aguiero VM (1997) Population characteristics and tungro transmission by Nephotettix virescens (Hemiptera: Cicadellidea) on selected resistant rice cultivars. Bull Entomol Res 87:387–395
Dai S, Zheng P, Marmey P, Zhang S, Tian W, Chen S, Beachy RN, Fauquet C (2001) Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol Breed 7:25–33
Dasgupta I, Das BK, Nath SP, Mukhopadhyay S, Niaiz FR, Varma A (1996) Detection of rice tungro bacilliform virus in field and glasshouse samples from India using the polymerase chain reaction. J Virol Methods 58:53–58
Dasgupta I, Hull R, Eastop S, Poggi PC, Blakebrough M, Boulton MI, Davies JW (1991) Rice tungro bacilliform virus DNA independently infects rice after Agrobacterium mediated transfer. J Gen Virol 72:1215–1221
Dong J, Kharb P, Cervera M, Hall CT (2001) The use of FISH in chromosomal localization of transgenes in rice. Methods Cell Sci 23:105–113
Dutta I, Saha P, Majumder P, Sarkar A, Chakraborti D, Banerjee S, Das S (2005a) The efficacy of a novel insecticidal protein, Allium sativum leaf lectin (ASAL), against homopteran insects monitored in transgenic tobacco. Plant Biotechnol J 3:601–611
Dutta I, Majumder I, Saha P, Ray K, Das S (2005b) Constitutive and phloem specific expression of Allium sativum leaf agglutinin (ASAL) to engineer aphid (Lipaphis erysimi) resistance in transgenic Indian mustard (Brassica juncea). Plant Sci 169:996–1007
Eamens AL, Blanchard CL, Dennis ES, Upadhyaya NM (2004) A bidirectional gene trap construct for tDNA and Ds-mediated insertional mutagenesis in rice (Oryza sativa L.). Plant Biotechnol J 2:367–380
Eisemann CH, Donaldson RA, Pearson RD, Cadagon LC, Vuocolo T, Pellam RL (1994) Larvicidal action of lectins on Lucilia cuprina; mechanism of action. Entomol Exp Appl 72:1–11
Fagard M, Vaucheret H (2000) (Trans)gene siliencing in plants: How many mechanisms? Annu Rev Plant Physiol Plant Mol Biol 51:167–194
Fleet GH (1991) Cell walls. In: Harrison AHR, Harrison JS (Eds.), Yeast organelles, Academic Press, London, pp 199–277
Filichkin SA, Brumfield S, Filichkin TP, Young MJ (1997) In vitro interactions of the aphid endosymbiotic SymL chaperonin with barley yellow dwarf virus. J Virol 71:569–577
Finnegan J, McElory D (1994) Transgene inactivation: plant fight back! Bio/Technol 12:883–888
Fitches E, Woodhouse SD, Edwards JP, Gatehoush JA (2001) In vitro and in vivo binding of snowdrop lectin (Galanthus nivalis agglutinin; GNA) and jackbean (Canavalia ensiformis; Con A) lectins within tomato moth (Lacanobia oleraceae) larvae; mechanisms of insecticidal action. J Insect Physiol 47:777–787
Foissac X, Loc NT, Christou P, Gatehouse AMR, Gatehouse JA (2000) Resistance to green leafhopper (Nephotettix virescens) and brown planthopper (Nilaparvata lugens) in transgenic rice expressing snowdrop lectin (Galanthus nivalis agglutinin; GNA). J Insect Physiol 46:573–583
Froissart R, Michalakis Y, Blanc S (2002) Helper component-transcomplementation in the vector transmission of plant viruses. Phytopathol 92:576–579
Gatehouse AM, Davison GM, Stewart JN, Gatehouse LN, Kumar A, Geoghegan IE, Birch ANE, Gatehouse JA (1999) Concanavalin A inhibits development of tomato moth (Lacanobia oleracea) and peach-potato aphid (Myzus persicae) when expressed in transgenic potato plants. Mol Breed 5:153–165
Graham MW, Craig S, Waterhouse PM (1997) Expression patterns of vascular-specific promoter RolC and Sh in transgenic potatoes and their use in engineering PLRV-resistant plants. Plant Mol Biol 33:729–735
Hogenhout SA, van den Wilk F, Verbeek M, Goldbach RW, van den Heuvel JFJM (1998) Potato leafroll virus binds to the equatorial domain of the aphid endosymbiotic GroEL homolog. J Virol 72:358–365
Huet H, Mahendra S, Wang J, Sivamani E, Ong CA, Chen L, de Kochko A, Beachy RN, Fauquet C (1999) Near immunity to rice tungro spherical virus achieved in rice by a replicase-mediated resistance strategy. Phytopathol 89:1022–1027
Jacob SS, Valuthambi K (2003) A cointegrate Ti plasmid vector for Agrobacterium tumefaciens-mediated transformation of indiac rice cv Pusa Basmati1. J Plant Biochem Biotech 12:1–9
Jain RK, Jain S (2000) Transgenic strategies for genetic improvement of Basmati rice. Indian J Exp Biol 38:6–17
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jiang J, Gill BS, Wang G-L, Ronald CP, Ward DC (1995) Metaphase and interphase fluorescence in situ hybridization mapping of the rice genome with bacterial artificial chromosomes. Proc Natl Acad Sci USA 92:4487–4491
Jin WW, Li ZY, Fang Q, Altosaar I, Lui LH, Song YC (2002) Fluorescence in situ hybridization analysis of alien genes in Agrobacterium-mediated Cry1A (b)- transformed rice. Ann Bot 90:31–36
Jones MC, Gough K, Dasgupta I, Subba-Rao BL, Cliffe J, Qu R, Shen P, Kaniewska M, Blakebrough M, Davies JW, Beachy RN, Hull R (1991) Rice tungro disease is caused by an RNA and a DNA virus. J Gen Virol 72:757–761
Kim SR, Lee J, Jun SH, Park S, Kang HG, Kwon S, An G (2003) Transgene structures in T-DNA-inserted rice plants. Plant Mol Biol 52:761–773
Kononov ME, Bassuner B, Gelvin SB (1997) Integration of T-DNA binary vector ‘backbone’ sequences into the tobacco genome: evidence for multiple complex patterns of integration. Plant J 11:945–957
Kubaláková M, Vrana J, Číhalíková J, Lysák MA, Doležel J (2001) Localization of DNA sequences on plant chromosomes using PRINS and C-PRINS. Methods Cell Sci 23:71–82
Lee SI, Lee S-H, Koo JC, Chun HJ, Lim CO, Mun JH, Song YH, Cho MJ (1999) Soybean Kunitz trypsin inhibitor (SKTI) confers resistance to the brown planthoppe (Nilaparvata lugens Stal) in transgenic rice. Mol Breed 5:1–9
Majumder P, Banerjee S, Das S (2004) Identification of receptors responsible for binding of the mannose specific lectin to the gut epithelial membrane of the target insects. Glycoconjugate J 20:525–530
Matsuki R, Onodera H, Yamauchi T, Uchimiya H (1989) Tissue-specific expression of the rolC promoter of the Ri plasmid in transgenic rice plants. Mol Gen Genet 220:12–16
Mohanty A, Sarma NP, Tyagi AK (1999) Agrobacterium-mediated high frequency transformation of an elite indica rice variety Pusa Basmati 1 and transformation of the transgene to R2 progey. Plant Sci 147:127–137
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Plant Physiol 15:473–497
Muskens MWM, Vissers APA, Mol JNM, Kooter JM (2000) Role of inverted DNA repeats in transcriptional and post-transcriptional gene silencing. Plant Mol Biol 43:243–260
Nagadhara D, Ramesh S, Pasalu IC, Rao YK, Krishnaiah NV, Sarma NP, Brown DP, Gatehouse JA, Reddy VD, Rao KV (2003) Transgenic indica rice resistant to sap-sucking insects. Plant Biotechnol J 1:231–240
Nagadhara D, Ramesh S, Pasalu IC, Rao YK, Sarma NP, Reddy VD, Rao KV (2004) Transgenic rice plants expressing the snowdrop lectin (gna) exhibit high-level resistance to the whitebacked planthopper (Sogetella furcifera). Theor Appl Genet 109:1399–1405
Powell KS, Spence J, Bharathi M, Gatehouse AJ, Gatehouse AMR (1998) Immunohistochemical and developmental studies to elucidate the mechanism of action of the snowdrop lectin on the rice brown planthopper, Nilaparvata lugens (Stal). J Insect Physiol 44:529–539
Rao KV, Rathore KS, Hodges TK, Fu X, Stoger E, Sudhakar S, Williams P, Christou P, Bharathi M, Bown DP, Powell KS, Spence J, Gatehouse A, Gatehouse JA (1998) Expression of snowdrop lectin (GNA) in transgenic plants confers resistance to rice brown planthopper. Plant J 15:469– 477
Schmulling T, Schell J, Spena A (1989) Promoters of the rolA, B, and C genes of Agrobacterium rhizogenes are differentially regulated in transgenic plants. Plant Cell 1:665–670
Shi Y, Wang MB, Powell KS, Damme EV, Hilder VA, Gatehouse AMR, Boulter D, Gatehouse JA (1994) Use of the rice sucrose synthase-1 promoter to direct phloem-specific expression of β-glucuronidase and snowdrop lectin genes in transgenic tobacco plants. J Exp Bot 45:623– 631
Shing RK, Shing US, Khush GS, Rohilla R (2000) Genetics and biotechnology of quality traits in aromatic rices In: Shing RK, Shing US, Khush GS (Eds.), Aromatic rices, Science Publishers Inc, Enfield, NH, USA, pp 47–69
Shou H, Frame BR, Whitham SAWK (2004) Assessment of transgenic maize events produced by particle bombardment or Agrobacterium-mediated transformation. Mol Breed 13:201–208
Sivamani E, Huet H, Shen P, Ong CA, de-Kochko A, Fauquet C, Beachy RN (1999) Rice plants (Oryza sativa L.) containing Rice tungro spherical virus (RTSV) coat protein transgenes are resistant to virus infection. Mol Breed 5:177–185
Sudhakar D, Fu X, Stoger E, Williams S, Spence J, Brown DP, Bharathi M, Gatehouse JA, Christou P (1998) Expression and immunolocalization of the snowdrop lectin, GNA in transgenic rice plants. Transgenic Res 7:371–378
Sugaya S, Hayakawa K, Handa K, Uchimiya H (1989) Cell-specific expression of the rolC gene of the TL-DNA of Ri plasmid in transgenic tobacco plants. Plant Cell Physiol 30:649–653
Suh S-O, Noda H, Blackwell M (2001) Insect symbiosis: derivation of yeast-like endosymbionts within an entomopathogenic filamentous lineage. Mol Biol Evol 18:995–1000
Travella S, Ross SM, Harden J, Everett C, Snape JW, Harwood WA (2004) A comparison of transgenic barley lines produced by particle bombardment and Agrobacterium-mediated techniques. Plant Cell Rep 23:780–789
Vaucheret H, Fagard M (2001) Transcriptional gene silencing in plant: target, inducers and regulators. Trends Genet 17:29–35
Wang M, Boulter D, Gatehouse JA (1992) A complete sequence of the rice sucrose synthase-1 (RSs1) gene. Plant Mol Biol 19:881–885
Wenck A, Czako M, Kanevski I, Marton L (1997) Frequent collinear long transfer of DNA inclusive of the whole binary vector during Agrobacterium-mediated transformation. Plant Mol Biol 34:913–922
Weis WI, Drickaner K (1996) Structural basis of lectin-carbohydrate recognition. Annu Rev Biotechnol 65:441–473
Yin Y, Zhu Q, Dai S, Lamb C, Beachy RN (1997) RF2a, a bZIP transcriptional activator of the phloem-specific rice tungro bacilliform virus promoter, functions in vascular development. EMBO J 16:5247–5259
Acknowledgements
Authors are grateful to Council of Scientific and Industrial Research; Government of India for providing fellowships to PS. We are grateful to Prof. S. C. Roy, Centre of Advanced Study (CAS), Cell and Chromosome Research, Department of Botany, University of Calcutta, 35 Ballygunge Circular Road, Kolkata, India for his help to carry out C-PRINS study in his laboratory. Authors thank the Programme Coordinator, CAS, Dept. Bot. CU for providing above technical opportunities. We are thankful to Regional Rice Research Station, Chinsurah, West Bengal, India for providing nuclear stock seed of Pusa Basmati 1 rice cultivar. For back up service of Mr. Arup Kumar Dey, BI is sincerely acknowledged. Authors are also thankful to Gautam Basu for critically reading the manuscript. The expert technical help of Anand Singh Rana in viral assays and Trilok Singh Rawat in inoculations of DU(SC) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Saha, P., Dasgupta, I. & Das, S. A novel approach for developing resistance in rice against phloem limited viruses by antagonizing the phloem feeding hemipteran vectors. Plant Mol Biol 62, 735–752 (2006). https://doi.org/10.1007/s11103-006-9054-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-006-9054-6