Abstract
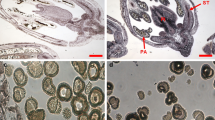
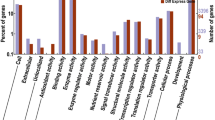
Elucidating the regulatory mechanisms of plant organ formation is an important component of plant developmental biology and will be useful for crop improvement applications. Plant organ formation, or organogenesis, occurs when a group of primordial cells differentiates into an organ, through a well-orchestrated series of events, with a given shape, structure and function. Research over the past two decades has elucidated the molecular mechanisms of organ identity and dorsalventral axis determinations. However, little is known about the molecular mechanisms underlying the successive processes. To develop an effective approach for studying organ formation at the molecular level, we generated organ-specific gene expression profiles (GEPs) reflecting early development in rice stamen. In this study, we demonstrated that the GEPs are highly correlated with early stamen development, suggesting that this analysis is useful for dissecting stamen development regulation. Based on the molecular and morphological correlation, we found that over 26 genes, that were preferentially up-regulated during early stamen development, may participate in stamen development regulation. In addition, we found that differentially expressed genes during early stamen development are clustered into two clades, suggesting that stamen development may comprise of two distinct phases of pattern formation and cellular differentiation. Moreover, the organ-specific quantitative changes in gene expression levels may play a critical role for regulating plant organ formation.
Similar content being viewed by others
Abbreviations
- aRNA:
-
antisense RNA
- cDNA:
-
complementary DNA
- DEPC:
-
diethyl pyrocarbonate
- dUTP:
-
deoxyuridine triphosphate
- EMS:
-
EXCESS MICROSPOROCYTES
- ESTs:
-
expression sequence tags
- EXS:
-
EXTRA SPOROGENOUS CELLS
- FIL:
-
FILAMENTOUS FLOWER
- LLS:
-
LETHAL LEAF SPOT
- LPA:
-
linear polyacrylamide
- msca1 :
-
male sterile converted anther1
- NZZ:
-
NOZZLE
- OsMADS7:
-
rice MADS-box protein 7
- PCR:
-
polymerase chain reaction
- PHD:
-
plant homeodomain
- RNA:
-
ribosome nucleic acid
- RT:
-
reverse transcription
- SPL:
-
SPOROCYTELESS
- TPD:
-
TAPETUM DETERMINANT
References
Bai SL, Peng YB, Cui JX, Gu HT, Xu LY, Li YQ, Xu ZH, Bai SN (2004) Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L). Planta 220:230–240
Baugh LR, Hill AA, Brown EL, Hunter CP (2001) Quantitative analysis of mRNA amplification by in vitro transcription. Nucleic Acids Res 29:E29
Bey M, Stuber K, Fellenberg K, Schwarz-Sommer Z, Sommer H, Saedler H, Zachgo S (2004) Characterization of antirrhinum petal development and identification of target genes of the class BMADS box gene DEFICIENS. Plant Cell 16:3197–3215
Canales C, Bhatt AM, Scott R, Dickinson H (2002) EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol 12:1718–1727
Chaubal R, Anderson JR, Trimnell MR, Fox TW, Albertsen MC, Bedinger P (2003) The transformation of anthers in the msca1 mutant of maize. Planta 216:778–788
Coen ES, Meyerowitz EM (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353:31–37
Coen E, Rolland-Lagan AG, Matthews M, Bangham JA, Prusinkiewicz P (2004) The genetics of geometry. Proc Natl Acad Sci USA 101:4728–4735
Coen ES, Carpenter R (1993) The metamorphosis of flowers. Plant Cell 5:1175–1181
Freeling M (1992) A conceptual framework for maize leaf development. Dev Biol 153:44–58
Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5:1217–1229
Kang HG, Seonghoe J, Chung JE, Cho YG, An G (1997) Characterization of two rice MADS box genes that control flowering time. Mol Cells 7:559–566
Kerr MK, Churchill GA (2001) Experimental design for gene expression microarrays. Biostatistics 2:183–201
Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB (1990) Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2:1201–1224
Liang YK, Wang Y, Zhang Y, Li SG, Lu XC, Li H, Zou C, Xu ZH, Bai SN (2003) OsSET1, a novel SET-domain containing gene from rice. J Exp Bot 54:1995–1996
Mao X, Cai T, Olyarchuk JG, Wei L (2005) Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 21:3787–3793
McConnell JR, Barton MK (1998) Leaf polarity and meristem formation in Arabidopsis. Development 125:2935–2942
Nonomura K, Miyoshi K, Eiguchi M, Suzuki T, Miyao A, Hirochika H, Kurata N (2003) The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell 15:1728–1739
Poethig RS (1997) Leaf morphogenesis in flowering plants. Plant Cell 9:1077–1087
Raghavan V (1988) Anther and pollen development in rice (Oryza sativa). Am J Bot 75:183–196
Sablowski RW, Meyerowitz EM (1998) A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 92:93–103
Sachs T (1969) Regeneration experiments on the determination of the form of leaves. Israel J Bot 18:21–30
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Sawa S, Watanabe K, Goto K, Liu YG, Shibata D, Kanaya E, Morita EH, Okada K (1999) FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev 13:1079–1088
Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K (1999) Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc Natl Acad Sci USA 96:11664–11669
Sorensen A, Guerineau F, Canales-Holzeis C, Dickinson HG, Scott RJ (2002) A novel extinction screen in Arabidopsis thaliana identifies mutant plants defective in early microsporangial development. Plant J 29:581–594
Sussex IM (1955b) Morphogenesis in Solanum tuberosem L: experimental investigation of leaf dorsiventrality and orientation in the juvenile shoot. Phytomorphology 5:286–300
Sussex IM (1955a) Morphogenesis in Solanum tuberosum L: apical structure and developmental pattern of the juvenile shoot. Phytomorphology 5:253–273
Tsukaya H (2003) Organ shape and size: a lesson from studies of leaf morphogenesis. Curr Opin Plant Biol 6:57–62
Waites R, Hudson A (1995) phantastica: a gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121:2143–2153
Wellmer F, Riechmann JL, Alves-Ferreira M, Meyerowitz EM (2004) Genome-wide analysis of spatial gene expression in Arabidopsis flowers. Plant Cell 16:1314–1326
Yang M, Wardzala E, Johal GS, Gray J (2004) The wound-inducible Lls1 gene from maize is an orthologue of the Arabidopsis Acd1 gene, and the LLS1 protein is present in non-photosynthetic tissues. Plant Mol Biol 54:175–91
Yang SL, Xie LF, Mao HZ, Puah CS, Yang WC, Jiang L, Sundaresan V, Ye D (2003) Tapetum determinant1 is required for cell specialization in the Arabidopsis anther. Plant Cell 15:2792–2804
Yang WC, Ye D, Xu J, Sundaresan V (1999) The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev 13:2108–2117
Zhao DZ, Wang GF, Speal B, Ma H (2002) The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev 16:2021–2031
Zik M, Irish VF (2003) Global identification of target genes regulated by APETALA3 and PISTILLATA floral homeotic gene action. Plant Cell 15:207–222
Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR Arabidopsis microarray database and analysis toolbox. Plant Physiol 136:2621–2632
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao-Chun Lu, Hua-Qin Gong contributed equally to this work.
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lu, XC., Gong, HQ., Huang, ML. et al. Molecular analysis of early rice stamen development using organ-specific gene expression profiling. Plant Mol Biol 61, 845–861 (2006). https://doi.org/10.1007/s11103-006-0054-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11103-006-0054-3