Abstract
Purpose
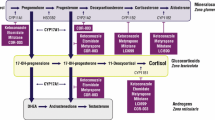
Corticotroph tumor progression (CTP) or Nelson’s syndrome (NS) can occur in patients with Cushing’s disease (CD) following bilateral adrenalectomy. It has rarely been observed in patients treated with long-term medical therapy for persistent CD. Osilodrostat (LCI699) is a new steroidogenesis inhibitor of 11β-hydroxylase (CYP11β1) that induced remission of hypercortisolism in 86% of patients with refractory CD in the randomized placebo-controlled trial LINC-3 (NCT02180217).
Methods
A 40-year-old woman with persistent CD following transsphenoidal surgery was treated with osilodrostat in the LINC-3 trial and was followed with regular hormonal assessments and imaging of residual corticotroph tumor.
Results
Under oral therapy with osilodrostat 10 mg twice daily, urinary free cortisol (UFC) normalized and clinical signs of CD regressed during therapy. However after 4 years of treatment, ACTH levels increased from 73 to 500 pmol/L and corticotroph tumor size increased rapidly from 3 to 14 mm, while UFCs remained well controlled. Surgical resection of an atypical tumor with weak ACTH expression and increased proliferative index (Ki-67 ≥ 8%) resulted in current remission but will require close follow-up.
Conclusion
This case highlights the importance of monitoring ACTH and corticotroph tumor size in patients with persistent CD, either under effective treatment with steroidogenesis inhibitors or after bilateral adrenalectomy.




Similar content being viewed by others
Data availability
Available from authors upon request.
References
Lacroix A, Feelders RA, Stratakis CA, Nieman LK (2015) Cushing’s syndrome. Lancet 386(9996):913–927. https://doi.org/10.1016/s0140-6736(14)61375-1
Fleseriu M, Findling JW, Koch CA, Schlaffer SM, Buchfelder M, Gross C (2014) Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing’s disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. J Clin Endocrinol Metab 99(10):3718–3727. https://doi.org/10.1210/jc.2014-1843
Feelders RA, Newell-Price J, Pivonello R, Nieman LK, Hofland LJ, Lacroix A (2019) Advances in the medical treatment of Cushing’s syndrome. Lancet Diabetes Endocrinol 7(4):300–312. https://doi.org/10.1016/s2213-8587(18)30155-4
Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A (2015) Treatment of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100(8):2807–2831. https://doi.org/10.1210/jc.2015-1818
Pivonello R, De Leo M, Cozzolino A, Colao A (2015) The treatment of cushing’s disease. Endocr Rev 36(4):385–486. https://doi.org/10.1210/er.2013-1048
Assié G, Bahurel H, Coste J, Silvera S, Kujas M, Dugué MA, Karray F, Dousset B, Bertherat J, Legmann P, Bertagna X (2007) Corticotroph tumor progression after adrenalectomy in Cushing’s Disease: A reappraisal of Nelson’s Syndrome. J Clin Endocrinol Metab 92(1):172–179. https://doi.org/10.1210/jc.2006-1328
Castinetti F, Morange I, Jaquet P, Conte-Devolx B, Brue T (2008) Ketoconazole revisited: a preoperative or postoperative treatment in Cushing’s disease. Eur J Endocrinol 158(1):91–99. https://doi.org/10.1530/eje-07-0514
Baudry C, Coste J, Bou Khalil R, Silvera S, Guignat L, Guibourdenche J, Abbas H, Legmann P, Bertagna X, Bertherat J (2012) Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol 167(4):473–481. https://doi.org/10.1530/eje-12-0358
Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A, Gu F, Auchus R, Leelawattana R, Lee EJ, Kim JH, Lacroix A, Laplanche A, O’Connell P, Tauchmanova L, Pedroncelli AM, Biller BMK (2020) Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre phase III study with a doubleblind, randomised withdrawal phase. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(20)30240-0
Jung C, Alford FP, Topliss DJ, Burgess JR, Long F, Gome JJ, Stockigt JR, Inder WJ (2010) The 4-mg intravenous dexamethasone suppression test in the diagnosis of Cushing’s syndrome. Clin Endocrinol (Oxf) 73(1):78–84. https://doi.org/10.1111/j.1365-2265.2009.03756.x
Vassiliadi DA, Tsagarakis S (2018) DIAgnosis of endocrine disease: the role of the desmopressin test in the diagnosis and follow-up of Cushing’s syndrome. Eur J Endocrinol 178(5):R201-r214. https://doi.org/10.1530/eje-18-0007
Nishioka H, Yamada S (2019) Cushing’s disease. J Clin Med. https://doi.org/10.3390/jcm8111951
Grossman AB (2017) The molecular pathology of cushing disease: are we nearly there? J Endocr Soc 1(2):144–148. https://doi.org/10.1210/js.2017-00036
Duggan S (2020) Osilodrostat: first approval. Drugs 80(5):495–500. https://doi.org/10.1007/s40265-020-01277-0
Fleseriu M, Pivonello R, Young J, Hamrahian AH, Molitch ME, Shimizu C, Tanaka T, Shimatsu A, White T, Hilliard A, Tian C, Sauter N, Biller BM, Bertagna X (2016) Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, Phase II study in Cushing’s disease. Pituitary 19(2):138–148. https://doi.org/10.1007/s11102-015-0692-z
Funding
No specific funding for this publication.
Author information
Authors and Affiliations
Contributions
CFS and AL designed the analysis, data collection and wrote the manuscript; LLG analyzed pituitary MRI imaging, RAM performed the pituitary surgeries and FB the pathology studies. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AL received funding as an investigator for participation in the LINC-3 trial from Novartis.
Ethical approval
LINC-3 study was approved by the CHUM ethics committee.
Informed consent
Patient provided informed signed consent to participate in the study and to publish results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fontaine-Sylvestre, C., Létourneau-Guillon, L., Moumdjian, R.A. et al. Corticotroph tumor progression during long-term therapy with osilodrostat in a patient with persistent Cushing’s disease. Pituitary 24, 207–215 (2021). https://doi.org/10.1007/s11102-020-01097-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-020-01097-1