Abstract
Purpose
Pasireotide is a multi-receptor-targeted somatostatin analogue approved in the EU and in the US for the treatment of adults with Cushing’s disease (CD). Pasireotide has a safety profile similar to other somatostatin analogues with the exception of hyperglycemia. In this report and literature review, the current understanding of predicting a positive treatment response to pasireotide in CD and the management of diabetes mellitus (DM) during pasireotide treatment are discussed and analyzed.
Case presentation
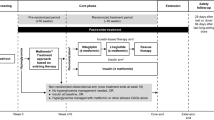
We report a case of a 55-year-old woman with CD and DM who benefitted from long-term pasireotide. The patient, who was enrolled in a phase III trial of the drug, showed early clinical improvements with pasireotide [900 μg subcutaneously twice daily (bid)] but was classified as a non-responder as urinary free cortisol (UFC) levels, were not normalized. Continuation of pasireotide for 12 months at an increased dose (1,200 μg bid) normalized UFC levels and restored cortisol rhythm. The initial deterioration in her blood glucose was managed with insulin and metformin; however, after 12 months’ treatment with pasireotide her DM was well controlled with oral hypoglycemic agents. Five years later, the patient is still receiving pasireotide (300 μg bid) with no loss of clinical or biochemical efficacy and with continued glycemic control.
Conclusions
This case presentation indicates that uncontrolled UFC levels during the first few months of pasireotide treatment as well as worsening of glycemic control in patients with CD and DM are not always predictive of the efficacy and tolerability and appears to support the long-term continuation of pasireotide.


Similar content being viewed by others
References
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462
Patil CG, Veeravagu A, Prevedello DM, Katznelson L, Vance ML, Laws ER Jr (2008) Outcomes after repeat transsphenoidal surgery for recurrent Cushing’s disease. Neurosurgery 63:266–270
Aghi MK (2008) Management of recurrent and refractory Cushing disease. Nat Clin Pract Endocrinol Metab 4:560–568
Mullan KR, Atkinson B (2008) Endocrine clinical update: Where are we in the therapeutic management of pituitary-dependent hypercortisolism? Clin Endocrinol 68:327–337
Arnaldi G, Boscaro M (2010) Pasireotide for the treatment of Cushing’s disease. Expert Opin Investig Drugs 19:889–898
Boscaro M, Ludlam WH, Atkinson B, Glusman JE, Petersenn S, Reincke M, Snyder P, Tabarin A, Biller BM, Findling J, Melmed S, Darby CH, Hu K, Wang Y, Freda PU, Grossman AB, Frohman LA, Bertherat J (2009) Treatment of pituitary dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 94:115–122
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM (2012) Pasireotide B2305 Study Group: a 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med 366:914–924
Pivonello, R., Petersenn, S., Newell-Price, J., Findling, J.W., Gu, F., Maldonado, M., Trovato, A., Hughes, G., Salgado, L.R., Lacroix, A., Schopohl, J., Biller, B.M.; Pasireotide B2305 Study Group. Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a Phase III study. Clin. Endocrinol. (Oxf) Feb 17, (2014) [Epub ahead of print]
Raff H (2013) Update on late-night salivary cortisol for the diagnosis of Cushing’s syndrome: methodological considerations. Endocrine 44(2):346–349
Colao, A., Boscaro, M., Ferone, D., Casanueva, F.F. Managing Cushing’s disease: the state of the art. Endocrine. Jan 11, (2014) [Epub ahead of print]
Trementino, L., Cardinaletti, M., Concettoni, C., Marcelli, G., Polenta, B., Spinello, M., Boscaro, M., Arnaldi, G. Salivary cortisol is a useful tool to assess the early response to pasireotide in patients with Cushing’s disease. Pituitary. Jan 31, (2014) [Epub ahead of print]
Petersenn S, Schopohl J, Barkan A, Mohideen P, Colao A, Abs R, Buchelt A, Ho YY, Hu K, Farrall AJ, Melmed S, Biller BM (2010) Pasireotide Acromegaly Study Group: pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, Phase II trial. J Clin Endocrinol Metab 95:2781–2789
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88:5593–5602
Arnaldi G, Mancini T, Tirabassi G, Trementino L, Boscaro M (2012) Advances in the epidemiology, pathogenesis, and management of Cushing’s syndrome complications. J Endocrinol Invest 35:434–448
Mazziotti G, Gazzaruso C, Giustina A (2011) Diabetes in Cushing syndrome: basic and clinical aspects. Trends Endocrinol Metab 22(12):499–506
Mazziotti G, Floriani I, Bonadonna S, Torri V, Chanson P, Giustina A (2009) Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. J Clin Endocrinol Metab 94(5):1500–1508
Shenouda, M., Maldonado, M., Wang, Y., Bouillaud, E., Hudson, M., Nesheiwat, D., Hu, K.: An open-label, dose-escalation study of once- and twice-daily pasireotide in healthy volunteers: Safety, tolerability and effects on glucose, insulin and glucagon levels. Am. J. Ther. Jun 16, (2012) [Epub ahead of print]
Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S (2013) Hyperglycemia associated with pasireotide:results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab 98(8):3446–3453
Colao, A., De Block, C., Gaztambide, M.S., Kumar, S., Seufert, J., Casanueva, F.F.: Managing hyperglycemia in patients with Cushing’s disease treated with pasireotide: medical expert recommendations. Pituitary. Apr 7, (2013) [Epub ahead of print]
Reznik Y, Bertherat J, Borson-Chazot F, Brue T, Chanson P, Cortet-Rudelli C, Delemer B, Tabarin A, Bisot-Locard S, Vergès B (2013) Management of hyperglycaemia in Cushing’s disease: experts’ proposals on the use of pasireotide. Diabetes Metab 39:34–41
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR, American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD) (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35(6):1364–1379
American Diabetes Association.: Standards of medical care in diabetes–2012. Diabetes. Care. 35 Suppl 1, S11–63 (2012)
Giordano R, Picu A, Marinazzo E, D’Angelo V, Berardelli R, Karamouzis I, Forno D, Zinnà D, Maccario M, Ghigo E, Arvat E (2011) Metabolic and cardiovascular outcomes in patients with Cushing’s syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol 75:354–360
Colao A, Pivonello R, Spiezia S, Faggiano A, Ferone D, Filippella M, Marzullo P, Cerbone G, Siciliani M, Lombardi G (1999) Persistence of increased cardiovascular risk in patients with Cushing’s disease after five years of successful cure. J Clin Endocrinol Metab 84:2664–2672
Webb SM, Mo D, Lamberts SW, Melmed S, Cavagnini F, Pecori Giraldi F, Strasburger CJ, Zimmermann AG, Woodmansee WW, International HypoCCS Advisory Board (2010) Metabolic, cardiovascular, and cerebrovascular outcomes in growth hormone-deficient subjects with previous Cushing’s disease or non-functioning pituitary adenoma. J Clin Endocrinol Metab 95:630–638
Kola B, Christ-Crain M, Lolli F, Arnaldi G, Giacchetti G, Boscaro M, Grossman AB, Korbonits M (2008) Changes in adenosine 5′-monophosphate-activated protein kinase as a mechanism of visceral obesity in Cushing’s syndrome. J Clin Endocrinol Metab 93:4969–4973
Cozzi R, Attanasio R, Montini M, Pagani G, Lasio G, Lodrini S, Barausse M, Albizzi M, Dallabonzana D, Pedroncelli AM (2003) Four-year treatment with octreotide-long-acting repeatable in 110 acromegalic patients: predictive value of short-term results? J Clin Endocrinol Metab 88:3090–3098
Fiorentino C, Motta C, Wolosinska DT, Monti S, Mori F, Toscano V (2011) SOM230 in Cushing’s disease complicated by poorly controlled diabetes mellitus. J Endocrinol Invest 34(9):731–732
Libé, R., Groussin, L., Bertherat, J.: Pasireotide in Cushing’s disease. N. Engl. J. Med. 366(22), 2134; author reply 2134–2135 (2012)
Shimon I, Rot L, Inbar E (2012) Pituitary-directed medical therapy with pasireotide for a orticotroph macroadenoma: pituitary volume reduction and literature review. Pituitary 15(4):608–613
Boscaro, M., Bertherat, J., Findling, J., Fleseriu, M., Atkinson, A.B., Petersenn, S., Schopohl, J., Snyder, P., Hughes, G., Trovato, A., Hu, K., Maldonado, M., Biller, B.M.: Extended treatment of Cushing’s disease with pasireotide: results from a 2-year, Phase II study. Pituitary. Aug 14, (2013) [Epub ahead of print]
Yedinak C, Brzana J, Fleseriu M (2013) Monitoring patient improvement parameters following pasireotide treatment in Cushing’s Disease. Case Rep Endocrinol 2013:735489
Lu L, Duan L, Jin Z, Lu Z, Gu F (2013) Effective long-term treatment of Cushing’s disease with pasireotide: a case report. Endocr Pract 19(4):e92–e96
Mackenzie Feder, J., Bourdeau, I., Vallette, S., Beauregard, H., Ste-Marie, L.G., Lacroix, A.: Pasireotide monotherapy in Cushing’s disease: a single-centre experience with 5-year extension of phase III Trial. Pituitary. Nov 28, 2013 [Epub ahead of print]
Acknowledgments
The authors would like to thank Max Chang of in Science Communications, Springer Healthcare for the editorial assistance. The authors received editorial/writing support in the preparation of this manuscript provided by in Science Communications, Springer Healthcare. This support was funded by Novartis, Italy. The authors did not receive honoraria related to the preparation of this manuscript.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trementino, L., Cardinaletti, M., Concettoni, C. et al. Up-to 5-year efficacy of pasireotide in a patient with Cushing’s disease and pre-existing diabetes: literature review and clinical practice considerations. Pituitary 18, 359–365 (2015). https://doi.org/10.1007/s11102-014-0582-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0582-9