Abstract
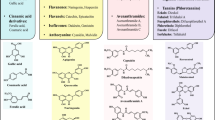
Gut microbiota contribute to the metabolism of dietary polyphenols and affect the bioavailability of both the parent polyphenols and their metabolites. Although there is a large number of reports of specific polyphenol metabolites, relatively little is known regarding the chemistry and enzymology of the metabolic pathways utilized by specific microbial species and taxa, which is the focus of this review. Major classes of dietary polyphenols include monomeric and oligomeric catechins (proanthocyanidins), flavonols, flavanones, ellagitannins, and isoflavones. Gut microbial metabolism of representatives of these polyphenol classes can be classified as A- and C-ring cleavage (retro Claisen reactions), C-ring cleavage mediated by dioxygenases, dehydroxylations (decarboxylation or reduction reactions followed by release of H2O molecules), and hydrogenations of alkene moieties in polyphenols, such as resveratrol, curcumin, and isoflavones (mediated by NADPH-dependent reductases). The qualitative and quantitative metabolic output of the gut microbiota depends to a large extent on the metabolic capacity of individual taxa, which emphasizes the need for assessment of functional analysis in conjunction with determinations of gut microbiota compositions.














Similar content being viewed by others
References
Amin HP, Czank C, Raheem S et al (2015) Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol Nutr Food Res 59:1095–1106
Appeldoorn MM, Vincken JP, Aura AM et al (2009) Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J Agric Food Chem 57:1084–1092
Aura AM, Martin-Lopez P, O’Leary KA et al (2005) In vitro metabolism of anthocyanins by human gut microflora. Eur J Nutr 44:133–142
Barroso E, Sanchez-Patan F, Martin-Alvarez PJ et al (2013) Lactobacillus plantarum IFPL935 favors the initial metabolism of red wine polyphenols when added to a colonic microbiota. J Agric Food Chem 61:10163–10172
Bitsch I, Janssen M, Netzel M et al (2004) Bioavailability of anthocyanidin-3-glycosides following consumption of elderberry extract and blackcurrant juice. Int J Clin Pharmacol Ther 42:293–300
Bode LM, Bunzel D, Huch M et al (2013) In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr 97:295–309
Bottiglieri M, Keel C (2006) Characterization of PhlG, a hydrolase that specifically degrades the antifungal compound 2,4-diacetylphloroglucinol in the biocontrol agent Pseudomonas fluorescens CHA0. Appl Environ Microbiol 72:418–427
Bowater L, Fairhurst SA, Just VJ et al (2004) Bacillus subtilis YxaG is a novel Fe-containing quercetin 2,3-dioxygenase. FEBS Lett 557:45–48
Bowey E, Adlercreutz H, Rowland I (2003) Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol 41:631–636
Brzezinski A, Debi A (1999) Phytoestrogens: the “natural” selective estrogen receptor modulators? Eur J Obstet Gynecol Reprod Biol 85:47–51
Bub A, Watzl B, Heeb D et al (2001) Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur J Nutr 40:113–120
Buckel W, Golding BT (2006) Radical enzymes in anaerobes. Annu Rev Microbiol 60:27–49
Cao G, Prior RL (1998) Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem 44:1309–1315
Carmona M, Zamarro MT, Blazquez B et al (2009) Anaerobic Catabolism of Aromatic Compounds: a Genetic and Genomic View. Microbiol Mol Biol Rev 73:71
Chadwick RW, George SE, Claxton LD (1992) Role of the gastrointestinal mucosa and microflora in the bioactivation of dietary and environmental mutagens or carcinogens. Drug Metab Rev 24:425–492
Czank C, Cassidy A, Zhang Q et al (2013) Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13) C-tracer study. Am J Clin Nutr 97:995–1003
Donovan JL, Manach C, Rios L et al (2002) Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br J Nutr 87:299–306
Eggler AL, Gay KA, Mesecar AD (2008) Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res 52:S84–S94
Eggler AL, Small E, Hannink M et al (2009) Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. Biochem J 422:171–180
Felgines C, Talavera S, Gonthier MP et al (2003) Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J Nutr 133:1296–1301
Fetzner S (2012) Ring-cleaving dioxygenases with a cupin fold. Appl Environ Microbiol 78:2505–2514
Gall M, Thomsen M, Peters C et al (2014) Enzymatic conversion of flavonoids using bacterial chalcone isomerase and enoate reductase. Angew Chem Int Ed Engl 53:1439–1442
Garcia-Villalba R, Beltran D, Espin JC et al (2013) Time course production of urolithins from ellagic acid by human gut microbiota. J Agric Food Chem 61:8797–8806
Gardana C, Canzi E, Simonetti P (2014) R(–)–O–desmethylangolensin is the main enantiomeric form of daidzein metabolite produced by human in vitro and in vivo. J Chromatogr B Analyt Technol Biomed Life Sci 953–954:30–37
Gonthier MP, Cheynier V, Donovan JL et al (2003) Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J Nutr 133:461–467
Gonzalez-Sarrias A, Gimenez-Bastida JA, Garcia-Conesa MT et al (2010) Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res 54:311–322
Goodrich KM, Neilson AP (2014) Simultaneous UPLC-MS/MS analysis of native catechins and procyanidins and their microbial metabolites in intestinal contents and tissues of male Wistar Furth inbred rats. J Chromatogr B Analyt Technol Biomed Life Sci 958:63–74
Gray NE, Sampath H, Zweig JA et al (2015) Centella asiatica attenuates amyloid-beta-induced oxidative stress and mitochondrial dysfunction. J Alzheimers Dis 45:933–946
Gu L, Kelm MA, Hammerstone JF et al (2004) Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr 134:613–617
Hanske L, Loh G, Sczesny S et al (2009) The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J Nutr 139:1095–1102
Hanske L, Loh G, Sczesny S et al (2010) Recovery and metabolism of xanthohumol in germ-free and human microbiota-associated rats. Mol Nutr Food Res 54:1405–1413
Harini R, Pugalendi KV (2010) Antihyperglycemic effect of protocatechuic acid on streptozotocin-diabetic rats. J Basic Clin Physiol Pharmacol 21:79–91
Hassaninasab A, Hashimoto Y, Tomita-Yokotani K et al (2011) Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc Natl Acad Sci USA 108:6615–6620
He YX, Huang L, Xue Y et al (2010) Crystal structure and computational analyses provide insights into the catalytic mechanism of 2,4-diacetylphloroglucinol hydrolase PhlG from Pseudomonas fluorescens. J Biol Chem 285:4603–4611
Hein EM, Rose K, van’t Slot G et al (2008) Deconjugation and degradation of flavonol glycosides by pig cecal microbiota characterized by Fluorescence in situ hybridization (FISH). J Agric Food Chem 56:2281–2290
Heinken A, Thiele I (2015) Anoxic conditions promote species-specific mutualism between gut microbes in silico. Appl Environ Microbiol 81:4049–4061
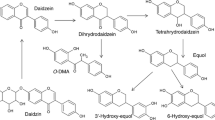
Heinonen S-M, Hoikkala A, Wähälä K et al (2003) Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects: identification of new metabolites having an intact isoflavonoid skeleton. J Steroid Biochem Mol Biol 87:285–299
Herles C, Braune A, Blaut M (2004) First bacterial chalcone isomerase isolated from Eubacterium ramulus. Arch Microbiol 181:428–434
Hirooka K, Fujita Y (2010) excess production of bacillus subtilis quercetin 2,3-dioxygenase affects cell viability in the presence of quercetin. Biosci Biotechnol Biochem 74:1030–1038
Hoffmann T, Troup B, Szabo A et al (1995) The anaerobic life of Bacillus subtilis: cloning of the genes encoding the respiratory nitrate reductase system. FEMS Microbiol Lett 131:219–225
Holder GM, Plummer JL, Ryan AJ (1978) The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 8:761–768
Hong HA, Khaneja R, Tam NM et al (2009) Bacillus subtilis isolated from the human gastrointestinal tract. Res Microbiol 160:134–143
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Hur HG, Beger RD, Heinze TM et al (2002) Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch Microbiol 178:8–12
Ichiyanagi T, Shida Y, Rahman MM et al (2008) Effect on both aglycone and sugar moiety towards Phase II metabolism of anthocyanins. Food Chem 110:493–500
Ireson CR, Jones DJ, Orr S et al (2002) Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomark Prev 11:105–111
Jimenez-Giron A, Ibanez C, Cifuentes A et al (2015) Faecal metabolomic fingerprint after moderate consumption of red wine by healthy subjects. J Proteome Res 14:897–905
Joannou GE, Kelly GE, Reeder AY et al (1995) A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J Steroid Biochem Mol Biol 54:167–184
Kakkar S, Bais S (2014) A review on protocatechuic Acid and its pharmacological potential. ISRN Pharmacol 2014:952943
Kang S, Joo C, Kim SM et al (2011) Oxidation of benzoins to benzoic acids using sodium hydride under oxygen atmosphere. Tetrahedron Lett 52:502–504
Karplus PA, Fox KM, Massey V (1995) Flavoprotein structure and mechanism. 8. Structure-function relations for old yellow enzyme. FASEB J 9:1518–1526
Kavanagh KL, Jornvall H, Persson B et al (2008) Medium- and short-chain dehydrogenase/reductase gene and protein families: the SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci 65:3895–3906
Kay CD, Mazza G, Holub BJ et al (2004) Anthocyanin metabolites in human urine and serum. Br J Nutr 91:933–942
Kim M, Han J (2014) Chiroptical study and absolute configuration of (–)–O–DMA produced from daidzein metabolism. Chirality 26:434–437
Kim M, Kim SI, Han J et al (2009) Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl Environ Microbiol 75:3062–3068
Kim M, Marsh EN, Kim SU et al (2010) Conversion of (3S,4R)-tetrahydrodaidzein to (3S)-equol by THD reductase: proposed mechanism involving a radical intermediate. Biochemistry 49:5582–5587
Knights D, Ward TL, McKinlay CE et al (2014) Rethinking “enterotypes”. Cell Host Microbe 16:433–437
Kumar H, Kim I-S, More SV et al (2014) Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep 31:109–139
Lee I-S, Lim J, Gal J et al (2011) Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem Int 58:153–160
Lee-Hilz YY, Boerboom AM, Westphal AH et al (2006) Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem Res Toxicol 19:1499–1505
Legette L, Ma L, Reed RL et al (2012) Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol Nutr Food Res 56:466–474
Legette LL, Luna AY, Reed RL et al (2013) Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 91:236–241
Legette LL, Prasain J, King J et al (2014) Pharmacokinetics of equol, a soy isoflavone metabolite, changes with the form of equol (dietary versus intestinal production) in ovariectomized rats. J Agric Food Chem 62:1294–1300
Lin HH, Chen JH, Chou FP et al (2011) Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-kappaB pathway and MMP-2 production by targeting RhoB activation. Br J Pharmacol 162:237–254
Ma Z-C, Hong Q, Wang Y-G et al (2010) ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol Pharm Bull 33:29–34
Margalef M, Pons Z, Muguerza B et al (2014) A rapid method to determine colonic microbial metabolites derived from grape flavanols in rat plasma by liquid chromatography-tandem mass spectrometry. J Agric Food Chem 62:7698–7706
Maruo T, Sakamoto M, Ito C et al (2008) Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol 58:1221–1227
Masella R, Santangelo C, D’Archivio M et al (2012) Protocatechuic acid and human disease prevention: biological activities and molecular mechanisms. Curr Med Chem 19:2901–2917
Matthies A, Loh G, Blaut M et al (2012) Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal Slackia isoflavoniconvertens in gnotobiotic rats. J Nutr 142:40–46
McInerney MJ, Gieg LM (2004) An overview of anaerobic metabolism. In: Nakano MM, Zuber P (eds) Strict and facultatie anaerobes: medical and environmental aspects. Horiz Biosci, Norfolk, pp 27–66
Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P et al (2008) Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem 56:7796–7802
Milligan SR, Kalita JC, Pocock V et al (2000) The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J Clin Endocrinol Metab 85:4912–4915
Mulek M, Hogger P (2015) Highly sensitive analysis of polyphenols and their metabolites in human blood cells using dispersive SPE extraction and LC-MS/MS. Anal Bioanal Chem 407:1885–1899
Nunez-Sanchez MA, Garcia-Villalba R, Monedero-Saiz T et al (2014) Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res 58:1199–1211
Oppermann U, Filling C, Hult M et al (2003) Short-chain dehydrogenases/reductases (SDR): the 2002 update. Chem Biol Interact 143–144:247–253
Peiffer DS, Zimmerman NP, Wang LS et al (2014) Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev Res (Phila) 7:574–584
Peng X, Zhang Z, Zhang N et al (2014) In vitro catabolism of quercetin by human fecal bacteria and the antioxidant capacity of its catabolites. Food Nutr Res 58:23406
Pompella A, Sies H, Wacker R et al (2014) The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition 30:791–793
Possemiers S, Heyerick A, Robbens V et al (2005) Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J Agric Food Chem 53:6281–6288
Possemiers S, Bolca S, Grootaert C et al (2006) The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J Nutr 136:1862–1867
Rafii F (2015) The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 5:56–73
Rasmussen SE, Frederiksen H, Struntze Krogholm K et al (2005) Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol Nutr Food Res 49:159–174
Rechner AR, Smith MA, Kuhnle G et al (2004) Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med 36:212–225
Schaab MR, Barney BM, Francisco WA (2006) Kinetic and spectroscopic studies on the quercetin 2,3-dioxygenase from Bacillus subtilis. Biochemistry 45:1009–1016
Schneider H, Simmering R, Hartmann L et al (2000) Degradation of quercetin-3-glucoside in gnotobiotic rats associated with human intestinal bacteria. J Appl Microbiol 89:1027–1037
Schnider-Keel U, Seematter A, Maurhofer M et al (2000) Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J Bacteriol 182:1215–1225
Schoefer L, Mohan R, Braune A et al (2002) Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol Lett 208:197–202
Schoefer L, Braune A, Blaut M (2004) Cloning and expression of a phloretin hydrolase gene from Eubacterium ramulus and characterization of the recombinant enzyme. Appl Environ Microbiol 70:6131–6137
Schroder C, Matthies A, Engst W et al (2013) Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens. Appl Environ Microbiol 79:3494–3502
Selma MV, Beltran D, Garcia-Villalba R et al (2014a) Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct 5:1779–1784
Selma MV, Tomas-Barberan FA, Beltran D et al (2014b) Gordonibacter urolithinfaciens sp. nov., a urolithin-producing bacterium isolated from the human gut. Int J Syst Evol Microbiol 64:2346–2352
Serra A, Macia A, Romero MP et al (2012) Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem 130:383–393
Setchell KD, Clerici C, Lephart ED et al (2005) S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 81:1072–1079
Shimada Y, Yasuda S, Takahashi M et al (2010) Cloning and expression of a novel NADP(H)-dependent daidzein reductase, an enzyme involved in the metabolism of daidzein, from equol-producing Lactococcus strain 20-92. Appl Environ Microbiol 76:5892–5901
Shimada Y, Takahashi M, Miyazawa N et al (2011) Identification of two novel reductases involved in equol biosynthesis in Lactococcus strain 20-92. J Mol Microbiol Biotechnol 21:160–172
Shimada Y, Takahashi M, Miyazawa N et al (2012) Identification of a novel dihydrodaidzein racemase essential for biosynthesis of equol from daidzein in Lactococcus sp. strain 20-92. Appl Environ Microbiol 78:4902–4907
Shisler KA, Broderick JB (2014) Glycyl radical activating enzymes: structure, mechanism, and substrate interactions. Arch Biochem Biophys 546:64–71
Silverman RB (2002) The organic chemistry of enzyme-catalyzed reactions. Academic Press, London
Spencer JP, Chaudry F, Pannala AS et al (2000) Decomposition of cocoa procyanidins in the gastric milieu. Biochem Biophys Res Commun 272:236–241
Steiner RA, Kalk KH, Dijkstra BW (2002) Anaerobic enzyme·substrate structures provide insight into the reaction mechanism of the copper-dependent quercetin 2,3-dioxygenase. Proc Natl Acad Sci 99:16625–16630
Sun YJ, Huang QQ, Li P et al (2015) Catalytic dioxygenation of flavonol by M-complexes (M=Mn, Fe, Co, Ni, Cu and Zn)—mimicking the M-substituted quercetin 2,3-dioxygenase. Dalton Trans 44:13926–13938
Takagaki A, Nanjo F (2013) Catabolism of (+)-catechin and (−)-epicatechin by rat intestinal microbiota. J Agric Food Chem 61:4927–4935
Taylor CT, Colgan SP (2007) Hypoxia and gastrointestinal disease. J Mol Med (Berl) 85:1295–1300
Thomsen M, Tuukkanen A, Dickerhoff J et al (2015) Structure and catalytic mechanism of the evolutionarily unique bacterial chalcone isomerase. Acta Crystallogr D Biol Crystallogr 71:907–917
Toh H, Oshima K, Suzuki T et al (2013) Complete genome sequence of the equol-producing bacterium adlercreutzia equolifaciens DSM 19450T. Genome Announc 1:e00742
Tomas-Barberan FA, Garcia-Villalba R, Gonzalez-Sarrias A et al (2014) Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem 62:6535–6538
Truchado P, Larrosa M, Garcia-Conesa MT et al (2012) Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J Agric Food Chem 60:5749–5754
Tsao SM, Hsia TC, Yin MC (2014) Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF-kappaB pathways. Nutr Cancer 66:1331–1341
Tsuji H, Moriyama K, Nomoto K et al (2010) Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch Microbiol 192:279–287
Tsuji H, Moriyama K, Nomoto K et al (2012) Identification of an enzyme system for daidzein-to-equol conversion in Slackia sp. strain NATTS. Appl Environ Microbiol 78:1228–1236
Tulipani S, Urpi-Sarda M, Garcia-Villalba R et al (2012) Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J Agric Food Chem 60:8930–8940
Turnbaugh PJ, Ridaura VK, Faith JJ et al (2009) The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1:6ra14
Uchiyama S, Ueno T, Suzuki T (2013) Equol-producing lactic acid bacteria-containing composition. PCT/JP2004/009484
Ulbrich K, Reichardt N, Braune A et al (2015) The microbial degradation of onion flavonol glucosides and their roasting products by the human gut bacteria Eubacterium ramulus and Flavonifractor plautii. Food Res Int 67:349–355
van Duynhoven J, Vaughan EE, Jacobs DM et al (2011) Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci USA 108(Suppl 1):4531–4538
Varì R, D’Archivio M, Filesi C et al (2011) Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J Nutr Biochem 22:409–417
Wang LS, Stoner GD (2008) Anthocyanins and their role in cancer prevention. Cancer Lett 269:281–290
Wang XL, Kim KT, Lee JH et al (2004) C-ring cleavage of isoflavones daidzein and genistein by a newly-isolated human intestinal bacterium Eubacterium ramulus Julong 601. J Microbiol Biotechnol 14:766–771
Wang D, Xia M, Yan X et al (2012) Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res 111:967–981
Williamson G, Clifford MN (2010) Colonic metabolites of berry polyphenols: the missing link to biological activity? Br J Nutr 104(Suppl 3):S48–S66
Woodward GM, Needs PW, Kay CD (2011) Anthocyanin-derived phenolic acids form glucuronides following simulated gastrointestinal digestion and microsomal glucuronidation. Mol Nutr Food Res 55:378–386
Xu J, Gordon JI (2003) Honor thy symbionts. Proc Natl Acad Sci USA 100:10452–10459
Yin MC, Lin CC, Wu HC et al (2009) Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: potential mechanisms of action. J Agric Food Chem 57:6468–6473
Yokoyama S, Oshima K, Nomura I et al (2011) Complete genomic sequence of the equol-producing bacterium Eggerthella sp. strain YY7918, isolated from adult human intestine. J Bacteriol 193:5570–5571
Yuan JP, Wang JH, Liu X (2007) Metabolism of dietary soy isoflavones to equol by human intestinal microflora–implications for health. Mol Nutr Food Res 51:765–781
Acknowledgments
The authors are in part supported by National Institutes of Health Grant No. R01AT009168.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stevens, J.F., Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem Rev 15, 425–444 (2016). https://doi.org/10.1007/s11101-016-9459-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-016-9459-z