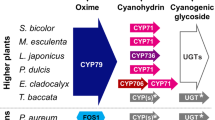
Abstract
Cyanogenic glycosides are ancient biomolecules found in more than 2,650 higher plant species as well as in a few arthropod species. Cyanogenic glycosides are amino acid-derived β-glycosides of α-hydroxynitriles. In analogy to cyanogenic plants, cyanogenic arthropods may use cyanogenic glycosides as defence compounds. Many of these arthropod species have been shown to de novo synthesize cyanogenic glycosides by biochemical pathways that involve identical intermediates to those known from plants, while the ability to sequester cyanogenic glycosides appears to be restricted to Lepidopteran species. In plants, two atypical multifunctional cytochromes P450 and a soluble family 1 glycosyltransferase form a metabolon to facilitate channelling of the otherwise toxic and reactive intermediates to the end product in the pathway, the cyanogenic glycoside. The glucosinolate pathway present in Brassicales and the pathway for cyanoalk(en)yl glucoside synthesis such as rhodiocyanosides A and D in Lotus japonicus exemplify how cytochromes P450 in the course of evolution may be recruited for novel pathways. The use of metabolic engineering using cytochromes P450 involved in biosynthesis of cyanogenic glycosides allows for the generation of acyanogenic cassava plants or cyanogenic Arabidopsis thaliana plants as well as L. japonicus and A. thaliana plants with altered cyanogenic, cyanoalkenyl or glucosinolate profiles.









Similar content being viewed by others
References
Andersen MD, Busk PK, Svendsen I, Møller BL (2000) Cytochromes P-450 from cassava (Manihot esculenta Crantz) catalyzing the first steps in the biosynthesis of the cyanogenic glucosides linamarin and lotaustralin—cloning, functional expression in Pichia pastoris, and substrate specificity of the isolated recombinant enzymes. J Biol Chem 275:1966–1975
Bak S, Feyereisen R (2001) The involvement of two P450 enzymes, CYP83B1 and CYP83A1, in auxin homeostasis and glucosinolate biosynthesis. Plant Physiol 127:108–118
Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA (1998a) Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol 36:393–405
Bak S, Nielsen HL, Halkier BA (1998b) The presence of CYP79 homologues in glucosinolate-producing plants shows evolutionary conservation of the enzymes in the conversion of amino acid to aldoxime in the biosynthesis of cyanogenic glucosides and glucosinolates. Plant Mol Biol 38:725–734
Bak S, Olsen CE, Petersen BL, Møller BL, Halkier BA (1999) Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J 20:663–671
Bak S, Olsen CE, Halkier BA, Møller BL (2000) Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Physiol 123:1437–1448
Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R (2001) CYP83B1, a cytochrome P450 at the metabolic branch paint in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell 13:101–111
Bayburt TH, Sligar SG (2003) Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Sci 12:2476–2481
Busk PK, Møller BL (2002) Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiol 129:1222–1231
Conn EE (1981) Cyanogenic glycosides. The biochemistry of plants, vol 7. Academic, New York, pp 479–500
Davis RH, Nahrstedt A (1987) Biosynthesis of cyanogenic glucosides in butterflies and moths—effective incorporation of 2-methylpropanenitrile and 2-methylbutanenitrile into linamarin and lotaustralin by Zygaena and Heliconius species (Lepidoptera). Insect Biochem 17:689–693
Douglas GC, Zipp BJ, Ludwig-Muller J, Masuno MN, Molinski TF, Abel S (2004) Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J 40:893–908
Duffey SS (1981) Cyanide and arthopods. In: Vennesland B, Conn EE, Knowles CJ, Westley J, Wissing F (eds) Cyanide in biology. Academic, London, pp 385–414
Duffey SS, Towers GHN (1978) Biochemical basis of HCN production in millipede Harpaphe haydeniana (Xystodesmidae: Polydesmida). Can J Zool 56:7–16
Engler HS, Spencer KC, Gilbert LE (2000) Insect metabolism—preventing cyanide release from leaves. Nature 406:144–145
Ettlinger MG, Kjær A (1968) Sulfur compounds in plants. Recent Adv Phytochem 1:49–144
Fang SD, Yan XQ, Li JF, Fan ZY, Xu XY, Xu Rs (1982) Studies on the chemical-constituents of Sedum sarmentosum bunge. IV. The structure of sarmentosin and iso-sarmentosin. Acta Chimi Sin 40:273–280
Feyereisen R (2005) Insect cytochrome P450. In: Gilbert LI, Latrou K, Gill SS (eds) Comprehensive molecular insect science, vol 4. Elsevier, Oxford, pp 1–77
Forslund K, Morant M, Jørgensen B, Olsen CE, Asamizu E, Sato S, Tabata S, Bak S (2004) Biosynthesis of the nitrile glucosides rhodiocyanoside A and D and the cyanogenic glucosides lotaustralin and linamarin in Lotus japonicus. Plant Physiol 135:71–84
Galbraith DW, Bak S (2005) Functional genomics of the cytochrome P450 gene superfamily in Arabidopsis thaliana. In: Leister D (ed) Plant functional genomics. Haworth, Binghamton, pp 595–620
Glawischnig E, Hansen BG, Olsen CE, Halkier BA (2004) Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc Natl Acad Sci USA 101:8245–8250
Gleadow RM, Woodrow IE (2000) Temporal and spatial variation in cyanogenic glycosides in Eucalyptus cladocalyx. Tree Physiol 20:591–598
Gleadow RM, Woodrow IE (2002) Defense chemistry of cyanogenic Eucalyptus cladocalyx seedlings is affected by water supply. Tree Physiol 22:939–945
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Halkier BA, Møller BL (1991) Involvement of cytochrome P-450 in the biosynthesis of dhurrin in Sorghum bicolor (L.) Moench. Plant Physiol 96:10–17
Halkier BA, Nielsen HL, Koch B, Møller BL (1995) Purification and characterization of recombinant cytochrome P450TYR expressed at high levels in Escherichia coli. Arch Biochem Biophys 322:369–377
Halkier BA, Sibbesen O, Møller BL (1996) Isolation of plant and recombinant CYP79. Methods Enzymol 272:268–274
Hansen CH, Du LC, Naur P, Olsen CE, Axelsen KB, Hick AJ, Pickett JA, Halkier BA (2001) CYP83B1 is the oxime-metabolizing enzyme in the glucosinolate pathway in Arabidopsis. J Biol Chem 276:24790–24796
Hansen KS, Kristensen C, Tattersall DB, Jones PR, Olsen CE, Bak S, Møller BL (2003) The in vitro substrate regiospecificity of recombinant UGT85B1, the cyanohydrin glucosyltransferase from Sorghum bicolor. Phytochemistry 64:143–151
Helliwell CA, Sheldon CC, Olive MR, Walker AR, Zeevaart JA, Peacock WJ, Dennis ES (1998) Cloning of the Arabidopsis ent-kaurene oxidase gene GA3. Proc Natl Acad Sci USA 95:9019–9024
Hemm MR, Ruegger MO, Chapple C (2003) The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell 15:179–194
Herrmann KM (1995) The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell 7:907–919
Holzkamp G, Nahrstedt A (1994) Biosynthesis of cyanogenic glucosides in the lepidoptera—incorporation of [U-C-14]-2-methylpropanealdoxime, 2S-[U-C-14]-methylbutanealdoxime and d,l-[U-C-14]-N-hydroxyisoleucine into linamarin and lotaustralin by the larvae of Zygaena trifolii. Insect Biochem Mol Biol 24:161–165
Humphreys JM, Chapple C (2002) Rewriting the lignin roadmap. Curr Opin Plant Biol 5:224–229
Jaroszewski JW, Olafsdottir ES, Wellendorph P, Christensen J, Franzyk H, Somanadhan B, Budnik BA, Jørgensen LB, Clausen V (2002a) Cyanohydrin glycosides of Passiflora: distribution pattern, a saturated cyclopentane derivative from P. guatemalensis, and formation of pseudocyanogenic alpha-hydroxyamides as isolation artefacts. Phytochemistry 59:501–511
Jaroszewski JW, Olafsdottir ES, Wellendorph P, Christensen J, Franzyk H, Somanadhan B, Budnik BA, Jørgensen LB, Clausen V (2002b) Natural cyclopentanoid cyanohydrin glycosides, part 23. Cyanohydrin glycosides of Passiflora: distribution pattern, a saturated cyclopentane derivative from P. guatemalensis, and formation of pseudocyanogenic alpha-hydroxyamides as isolation artefacts. Phytochemistry 59:501–511
Jones PR, Møller BL, Hoj PB (1999) The UDP-glucose: p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. Isolation, cloning, heterologous expression, and substrate specificity. J Biol Chem 274:35483–35491
Jones PR, Andersen MD, Nielsen JS, Høj PB, Møller BL (2000) The biosynthesis, degradation, transport and possible functions of cyanogenic glucosides. In: Romeo JT (eds) Recent advances in phytochemistry: evolution of metabolic pathways. Pergamon, Amsterdam, pp 191–247
Jørgensen K, Bak S, Busk PK, Sorensen C, Olsen CE, Puonti-Kaerlas J, Møller BL (2005a) Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Distribution of cyanogenic glucosides, their site of synthesis and transport, and blockage of the biosynthesis by RNA interference technology. Plant Physiol 139:363–374
Jørgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, Bak S, Møller BL (2005b) Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol 8:280–291
Kahn RA, Bak S, Svendsen I, Halkier BA, Møller BL (1997) Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol 115:1661–1670
Klein M, Reichelt M, Gershenzon J, Papenbrock J (2006) The three desulfoglucosinolate sulfotransferase proteins in Arabidopsis have different substrate specificities and are differentially expressed. FEBS J 273:122–136
Koch BM, Sibbesen O, Halkier BA, Svendsen I, Møller BL (1995) The primary sequence of cytochrome P450tyr, the multifunctional N-hydroxylase catalyzing the conversion of l-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys 323:177–186
Kristensen C, Morant M, Olsen CE, Ekstrom CT, Galbraith DW, Møller BL, Bak S (2005) Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proc Natl Acad Sci USA 102:1779–1784
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Kutchan TM (2005) Predictive metabolic engineering in plants: still full of surprises. Trends Biotechnol 23:381–383
Labandeira CC (1993) The real meaning of insect fossils. Palaios 8:509–511
Labandeira CC, Dilcher DL, Davis DR, Wagner DL (1994) 97-Million years of angiosperm–insect association—paleobiological insights into the meaning of coevolution. Proc Natl Acad Sci USA 91:12278–12282
Labandeira CC, Sepkoshi JJ Jr (1993) Insect diversity in the fossil record. Science 261:310–315
Lechtenberg M, Nahrstedt A (1999) Cyanogenic glycosides. In: Ikan R (ed) Naturally occurring glycosides. Wiley, New York, pp 147–191
Memelink J (2005) Tailoring the plant metabolome without a loose stitch. Trends Plant Sci 10:305–307
Mikkelsen MD, Halkier BA (2003) Metabolic engineering of valine- and isoleucine-derived glucosinolates in Arabidopsis expressing CYP79D2 from cassava. Plant Physiol 131:773–779
Mikkelsen MD, Petersen BL, Olsen CE, Halkier BA (2002) Biosynthesis and metabolic engineering of glucosinolates. Amino Acids 22:279–295
Mikkelsen MD, Naur P, Halkier BA (2004) Arabidopsis mutants in the C–S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J 37:770–777
Møller BL, Conn EE (1980) The biosynthesis of cyanogenic glucosides in higher plants. Channeling of intermediates in dhurrin biosynthesis by a microsomal system from Sorghum bicolor (linn) Moench. J Biol Chem 255:3049–3056
Møller BL, Seigler DS (1998) Biosynthesis of cyanogenic glucosides, cyanolipids, and related compounds. In: Singh BK (eds) Plant amino acids, biochemsitry and biotechnology. Marcel Dekker Inc., New York, pp 563–609
Morant M, Bak S, Møller BL, Werck-Reichhart D (2003) Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotechnol 14:151–162
Naur P, Petersen BL, Mikkelsen MD, Bak S, Rasmussen H, Olsen CE, Halkier BA (2003) CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol 133:63–72
Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW (1996) P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6:1–42
Nelson DR, Schuler MA, Paquette SM, Werck-Reichhart D, Bak S (2004) Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol 135:756–772
Nielsen JS, Møller BL (2000) Cloning and expression of cytochrome P450 enzymes catalyzing the conversion of tyrosine to p-hydroxyphenylacetaldoxime in the biosynthesis of cyanogenic glucosides in Triglochin maritima. Plant Physiol 122:1311–1321
Nielsen KA, Møller BL (2004) Cytochromes P450 in Plants. In: Ortiz de Montellano PR (ed) Cytochrome P450: structure, mechanism, and biochemistry, 3rd edn. Kluwer Academic/Plenum Publishers, New York, pp 553–583
Nielsen KA, Olsen CE, Pontoppidan K, Møller BL (2002) Leucine-derived cyano glucosides in barley. Plant Physiol 129:1066–1075
Paquette S, Møller BL, Bak S (2003) On the origin of the family 1 plant glycosyltransferases. Phytochemisty 62:399–413
Petersen BL, Andreasson E, Bak S, Agerbirk N, Halkier BA (2001) Characterization of transgenic Arabidopsis thaliana with metabolically engineered high levels of p-hydroxybenzylglucosinolate. Planta 212:612–618
Piotrowski M, Schemenewitz A, Lopukhina A, Muller A, Janowitz T, Weiler EW, Oecking C (2004) Desulfoglucosinolate sulfotransferases from Arabidopsis thaliana catalyze the final step in the biosynthesis of the glucosinolate core structure. J Biol Chem 279:50717–50725
Poulton J, Møller BL (1989) The biosynthesis of cyanogenic glucosides. In: Dey PM, Harborne JB (eds) Methods in plant biochemistry, vol 9. Academic, London, pp 183–207
Regier JC, Shultz JW, Kambic RE (2005) Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopoda are not monophyletic. Proc R Soc B 272:395–401
Rodman JE, Soltis PS, Soltis DE, Sytsma KJ, Karol KG (1998) Parallel evolution of glucosinolate biosynthesis inferred from congruent nuclear and plastid gene phylogenies. Am J Bot 85:997–1006
Schuhegger R, Nafisi M, Mansourova M, Petersen BL, Olsen CE, Svatos A, Halkier BA, Glawischnig E (2006) CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol 141:1248–1254
Scott EE, He YA, Wester MR, White MA, Chin CC, Halpert JR, Johnson EF, Stout CD (2003) An open conformation of mammalian cytochrome P4502B4 at 1.6-angstrom resolution. Proc Natl Acad Sci USA 100:13196–13201
Selmar D, Lieberei R, Biehl B (1988) Mobilization and utilization of cyanogenic glycosides: the linustatin pathway. Plant Physiol 86:711–716
Sibbesen O, Koch B, Halkier BA, Møller BL (1994) Isolation of the heme-thiolate enzyme cytochrome P-450TYR, which catalyzes the committed step in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Proc Natl Acad Sci USA 91:9740–9744
Sibbesen O, Koch B, Halkier BA, Møller BL (1995) Cytochrome P-450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of l-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. J Biol Chem 270:3506–3511
Siegler DS, Brinker AM (1993) Characterisation of cyanogenic glycosides, cyanolipids, nitroglycosides, organic nitro compounds and nitrile glycosides from plants. In: Dey PM, Harborne JB (eds) Methods of plant biochemistry, alkaloids and sulfur compounds. Academic, New York, pp 51–93
Siritunga D, Sayre RT (2003) Generation of cyanogen-free transgenic cassava. Planta 217:367–373
Siritunga D, Sayre R (2004) Engineering cyanogen synthesis and turnover in cassava (Manihot esculenta). Plant Mol Biol 56:661–669
Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Hoj PB, Møller BL (2001) Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293:1826–1828
Thorsoe KS, Bak S, Olsen CE, Imberty A, Breton C, Møller BL (2005) Determination of catalytic key amino acids and UDP sugar donor specificity of the cyanohydrin glycosyltransferase UGT85B1 from Sorghum bicolor. Molecular modeling substantiated by site-specific mutagenesis and biochemical analyses. Plant Physiol 139:664–673
Werck-Reichhart D, Bak S, Paquette SM (2002) Cytochrome P450. The Arabidopsis book. American Society of Plant Biologists, Rockville, pp 1–29
Williams PA, Cosme J, Ward A, Angova HC, Vinkovic DM, Jhoti H (2003) Crystal structure of human cytochrome P4502C9 with bound warfarin. Nature 424:464–468
Williams PA, Cosme J, Vinkovic DM, Ward A, Angove HC, Day PJ, Vonrhein C, Tickle IJ, Jhoti H (2004) Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science 305:683–686
Willis KJ, McElwain JC (2002) The evolution of plants. Oxford University Press Inc., New York
Winkel BS (2004) Metabolic channeling in plants. Annu Rev Plant Biol 55:85–107
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95:707–735
Yoshikawa M, Shimada H, Shimoda H, Matsuda H, Yamahara J, Murakami N (1995) Rhodiocyanoside-A and rhodiocyanoside-B, new antiallergic cyanoglycosides from Chinese natural medicine Si-Li-Hong-Jing-Tian, the underground part of Rhodiola quadrifida (Pall) Fisch et Mey. Chem Pharm Bull 43:1245–1247
Yoshikawa M, Shimada H, Matsuda H, Yamahara J, Murakami N (1996a) Bioactive constituents of Chinese natural medicines. 1. New sesquiterpene ketones with vasorelaxant effect from Chinese moxa, the processed leaves of Artemisia argyi Levl et Vant: moxartenone and moxartenolide. Chem Pharm Bull 44:1656–1662
Yoshikawa M, Shimada H, Shimoda H, Murakami N, Yamahara J, Matsuda H (1996b) Bioactive constituents of Chinese natural medicines. 2. Rhodiolae radix. 1. Chemical structures and antiallergic activity of rhodiocyanosides A and B from the underground part of Rhodiola quadrifida (Pall) Fisch et MEY (Crassulaceae). Chem Pharm Bull 44:2086–2091
Zagrobelny M, Bak S, Rasmussen AV, Jørgensen B, Naumann CM, Møller BL (2004) Cyanogenic glucosides and plant–insect interactions. Phytochemistry 65:293–306
Zagrobelny M, Bak S, Ekstrøm CT, Oslen CE, Møller BL: Cyanogenic glycosides in Zygaena filipendulae (Lepidoptera: Zygaenidae) as effected by feeding on wild-type and transgenic Lotus populations with variable cyanogenic profiles. Insect Biochemistry and Molecular Biology (in press)
Acknowledgements
Steen Malmmose, Susanne Jensen and Charlotte Sørensen are thanked for excellent technical assistance in the greenhouses and laboratories. All former members of the Cyanogenic Glycosides group are thanked for their contributions.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11101-007-9063-3.
Rights and permissions
About this article
Cite this article
Bak, S., Paquette, S.M., Morant, M. et al. Cyanogenic glycosides: a case study for evolution and application of cytochromes P450. Phytochem Rev 5, 309–329 (2006). https://doi.org/10.1007/s11101-006-9033-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-006-9033-1