Abstract
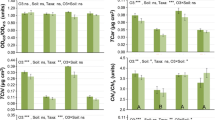
In this study, we questioned whether ground-level ozone (O3) induces hormesis in Japanese larch (Larix kaempferi) and its hybrid F1 (L. gmelinii var. japonica × L. kaempferi). In order to answer the question, we exposed seedlings of both taxa to four O3 treatments [ranging from ≈10 to 60 nmol(O3) mol–1] in open-top chambers for two consecutive growing seasons. We found a hormetic response in maximum photosynthetic rate (PNmax) at 1700 μmol(CO2) mol–1 and maximum rates of carboxylation (Vcmax) and electron transport (Jmax) in both larches. Stimulation of PNmax, Vcmax, and Jmax did not lead to suppressed plant productivity in Japanese larch, which followed a stress-tolerant strategy, but it did lead to suppressed plant productivity in hybrid larch which followed a competitive strategy. These findings are the first to suggest that stimulation of physiological functions by low O3 exposures may have negative consequences for larch reproduction.
Similar content being viewed by others
Abbreviations
- CF:
-
charcoal-filtered air
- Chl:
-
chlorophyll
- E :
-
transpiration rate
- g s :
-
stomatal conductance
- GLM:
-
general linear model
- HL:
-
hybrid larch
- HSD:
-
Tukey’s honest significance difference
- JL:
-
Japanese larch
- J max :
-
maximum electron transport rate
- NF:
-
nonfiltered air
- NF40:
-
NF enriched with O3 to reach 40 nmol mol–1
- NF60:
-
NF enriched with O3 to reach 60 nmol mol–1
- NOAEL:
-
no observed adverse effect level
- OTCs:
-
open-top chambers
- P N :
-
net photosynthetic rate
- P Nmax :
-
maximum photosynthetic rate at 1,700 μmol(CO2) mol–1
- RGR:
-
relative growth rate
- ROS:
-
reactive oxygen species
- V cmax :
-
maximum rate of carboxylation
- WUE:
-
water-use efficiency
References
Agathokleous E.: Perspectives for elucidating the ethylenediurea (EDU) mode of action for protection against O3 phytotoxicity. — Ecotox. Environ. Safe. 142: 530–537, 2017.
Agathokleous E., Saitanis C.J., Wang X. et al.: A review study on past 40 years of research on effects of tropospheric O3 on belowground structure, functioning, and processes of trees: a linkage with potential ecological implications. — Water Air Soil Poll. 227: 1–28, 2016a.
Agathokleous E., Saitanis C.J., Stamatelopoulos D. et al.: Olive oil for dressing plant leaves so as to avoid O3 injury. — Water Air Soil Poll. 227: 282, 2016b.
Agathokleous E., Watanabe M., Eguchi N. et al.: Root production of Fagus crenata blume saplings grown in two soils and exposed to elevated CO2 concentration: an 11-year freeair-CO2 enrichment (FACE) experiment in northern Japan. — Water Air Soil Poll. 227: 187, 2016c.
Agathokleous E., Vanderstock A., Kita K. et al.: Stem and crown growth of Japanese larch and its hybrid F1 grown in two soils and exposed to two free-air O3 regimes. — Environ. Sci. Pollut. R. 24: 6634–6647, 2017.
Ainsworth E.A., Yendrek C.R., Sitch S. et al.: The effects of tropospheric ozone on net primary productivity and implications for climate change. — Annu. Rev. Plant Biol. 63: 637–661, 2012.
Ainsworth E.A., Long S.P.: What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. — New Phytol. 165: 351–372, 2005.
Akimoto H., Mori Y., Sasaki K. et al.: Analysis of monitoring data of ground-level ozone in Japan for long-term trend during 1990–2010: causes of temporal and spatial variation. — Atmos. Environ. 102: 302–310, 2015.
Box G.E.P., Cox D.R.: An analysis of transformations. — J. Roy. Stat. Soc. B-Met 26: 211–252, 1964.
Calabrese E.J.: Biphasic dose responses in biology, toxicology and medicine: Accounting for their generalizability and quantitative features. — Environ. Pollut. 182: 452–460, 2013.
Calabrese E.J.: Hormesis: a fundamental concept in biology. — Microb. Cell 1: 145–149, 2014.
Calabrese E.J.: Hormesis: principles and applications. — Homeopathy 104: 69–82, 2015.
Calatayud V., Marco F., Cerveró J. et al.: Contrasting ozone sensitivity in related evergreen and deciduous shrubs. — Environ. Pollut. 158: 3580–3587, 2010.
Cedergreen N., Streibig J.C., Kudsk P. et al.: The occurrence of hormesis in plants and algae. — Dose-Response 5: 150–162, 2007.
Chatani S., Amann M., Goel A. et al.: Photochemical roles of rapid economic growth and potential abatement strategies on tropospheric ozone over South and East Asia in 2030. — Atmos. Chem. Phys. 14: 9259–9277, 2014.
Eamus D., Barnes J.D., Mortensen L. et al.: Persistent stimulation of CO2 assimilation and stomatal conductance by summer ozone fumigation in Norway spruce. — Environ. Pollut. 63: 365–379, 1990.
Farquhar G.V., von Caemmerer S.V., Berry J.A.: A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. — Planta 149: 78–90, 1980.
Feng Z., Hu E., Wang X. et al.: Ground-level O3 pollution and its impacts on food crops in China: a review. — Environ. Pollut. 199: 42–48, 2015.
Flowers M.D., Fiscus E.L., Burkey K.O. et al.: Photosynthesis, chlorophyll fluorescence, and yield of snap bean (Phaseolus vulgaris L.) genotypes differing in sensitivity to ozone. — Environ. Exp. Bot. 61: 190–198, 2007.
Fuhrer D., Skärby L., Ashmore M.R.R.: Critical levels for ozone effects on vegetation in Europe. — Environ. Pollut. 97: 91–106, 1997.
Gower S.T., Richards J.H.: Larches: deciduous conifers in an evergreen world. — BioScience 40: 818–826, 1990.
Grantz D.A., Gunn S., Vu H.B.: O3 impacts on plant development: a meta-analysis of root/shoot allocation and growth. — Plant Cell Environ. 29: 1193–1209, 2006.
Grime J.P.: Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. — Am. Nat. 111: 1169–1194, 1977.
Gyu K.D., Shi C., Watanabe M. et al.: Growth of Japanese and hybrid larch seedlings grown under free-air O3 fumigation — an initial assessment of the effects of adequate and excessive nitrogen. — J. Agr. Meteorol. 71: 239–244, 2015.
Hartmann D.L., Klein Tank A.M.G., Rusticucci M., et al.: Observations: atmosphere and surface. — In: Stocker T.F., Qin D., Plattner G.-K. et al. (ed.): Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, New York 2013.
Hoffmann W.A., Poorter H.: Avoiding bias in calculations of relative growth rate. — Ann. Bot.-London 90: 37–42, 2002.
Hopkins W.G.: A new view of statistics. — Internet Society for Sport Science. Available at http://newstats.org, 2000.
Hoshika Y., Watanabe M., Inada N. et al.: Model-based analysis of avoidance of ozone stress by stomatal closure in Siebold’s beech (Fagus crenata). — Ann. Bot.-London 112: 1149–1158, 2013.
IGBP: The terrestrial carbon cycle: Implication for the Kyoto Protocol. — Science 280: 1393–1394, 1998.
Kalabokas P.D., Thouret V., Cammas J.-P. et al.: The geographical distribution of meteorological parameters associated with high and low summer ozone levels in the lower troposphere and the boundary layer over the eastern Mediterranean (Cairo case). — Tellus B 67: 27853, 2015.
Kita K., Fujimoto T., Uchiyama K. et al.: Estimated amount of carbon accumulation of hybrid larch in three 31-year-old progeny test plantations. — J. Wood Sci. 55: 425–434, 2009.
Kitao M., Yasuda Y., Kominami Y. et al.: Increased phytotoxic O3 dose accelerates autumn senescence in an O3-sensitive beech forest even under the present-level O3. — Sci. Rep. 6: 32549, 2016.
Koike T., Mao Q., Inada N. et al.: Growth and photosynthetic responses of cuttings of a hybrid larch (Larix gmelinii var. japonica x L., kaempferi) to elevated ozone and/or carbon dioxide. — Asian J., Atmos. Environ. 6: 104–110, 2012.
Koike T., Watanabe M., Hoshika Y. et al.: Effects of ozone on forest ecosystems in East and Southeast Asia. — In: Matyssek R., Clarke N., Cudlin P., et al. (ed.): Climate Change, Air Pollution and Global Challenges: Understanding and Solutions from Forest Research, A COST Action. Pp. 371–390. Elsevier, Oxford 2013.
Küppers M.: Ecological significance of above-ground architectural patterns in woody plants: A question of costbenefit relationships. — Trends Ecol. Evol. 4: 375–379, 1989.
Kurinobu S.: Forest tree breeding for Japanese larch. — Eurasian J., For. Res. 8: 127–134, 2005.
Liancourt P., Callaway R.M., Michalet R.: Stress tolerance and competitive-response ability determine the outcome of biotic interactions. — Ecology 86: 1611–1618, 2005.
Long S.P., Bernacchi C.J.: Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. — J. Exp. Bot. 54: 2393–2401, 2003.
Manninen S., Huttunen S., Vanhatalo M. et al.: Inter-and intraspecific responses to elevated ozone and chamber climate in northern birches. — Environ. Pollut. 157: 1679–1688, 2009.
Matyssek R., Bytnerowicz A., Karlsson P.E. et al.: Promoting the ozone flux concept for European forest trees. — Environ. Pollut. 146: 587–607, 2007.
Matyssek R., Schulze E.-D.: Heterosis in hybrid larch (Larix decidua x L., leptolepis). — Trees 1: 225–231, 1987.
Osawa A., Zyryanova O.A., Matsuura Y. et al. (ed.): Permafrost Ecosystems: Siberian Larch Forests. — Ecological Studies 209. Pp. 502. Springer, Dordrecht 2010.
Paoletti E.: Impact of ozone on Mediterranean forests: A review. — Environ. Pollut. 144: 463–474, 2006.
Paoletti E., Manning W.J.: Toward a biologically significant and usable standard for ozone that will also protect plants. — Environ. Pollut. 150: 85–95, 2007.
Paoletti E., Ranieri A., Lauteri M.: Moving toward effective ozone flux assessment. — Environ. Pollut. 156: 16–19, 2008.
Pell E.J., Temple P.J., Friend A.L. et al.: Compensation as a plant response to ozone and associated stresses: an analysis of ROPIS experiments. — J. Environ. Qual. 23: 429–436, 1994.
Qu L.: Ecophysiological study on the natural regeneration of the two larch species with special references to soil environment in larch forests. — Eurasian J., For. Res. 19: 1–51, 2016.
Ryu K., Watanabe M., Shibata H. et al.: Ecophysiological responses of the larch species in northern Japan to environmental changes as a basis for afforestation. — Landsc. Ecol. Eng. 5: 99–106, 2009.
Schneider C.A., Rasband W.S., Eliceiri K.W.: NIH Image to ImageJ: 25 years of image analysis. — Nat. Methods 9: 671–675, 2012.
Šesták Z., (ed.): Photosynthesis during Leaf Development. Pp. 396. Springer, Dordrecht, Boston 1985.
Shinano T., Lei T.T., Kawamukai T. et al.: Dimethylsulfoxide method for the extraction of chlorophylls a and b from the leaves of wheat, field bean, dwarf bamboo, and oak. — Photosynthetica 32: 409–415, 1996.
Sicard P., Serra R., Rossello P.: Spatiotemporal trends in groundlevel ozone concentrations and metrics in France over the time period 1999–2012. — Environ. Res. 149: 122–144, 2016.
Vázquez-Ybarra J.A., Peña-Valdivia C.B., Trejo C. et al.: Promoting growth of lettuce plants (Lactuca sativa L.) with sublethal ozone doses applied to culture medium. — Rev. Fitotec. Mex. 38: 405–413, 2015.
Verstraeten W.W., Neu J.L., Williams J.E. et al.: Rapid increases in tropospheric ozone production and export from China. — Nature Geosci. 8: 690–695, 2015.
Whitehead F.H., Myerscough P.J.: Growth analysis of plants. The ratio of mean relative growth rate to mean relative rate of leaf area increase. — New Phytol. 61: 314–321, 1962.
Yamaguchi M., Watanabe M., Matsumura H. et al.: Experimental studies on the effects of ozone on growth and photosynthetic activity of Japanese forest tree species. — Asian J., Atmos. Environ. 5: 65–78, 2011.
Yendrek C.R., Leisner C.P., Ainsworth E.A.: Chronic ozone exacerbates the reduction in photosynthesis and acceleration of senescence caused by limited N availability in Nicotiana sylvestris. — Glob. Change Biol. 19: 3155–3166, 2013.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Acknowledgements: This study was supported by a Type B Grant-in-Aid (No. 26292075, to T. Koike) from Japan Society for the Promotion of Science (JSPS). E. Agathokleous is a JSPS International Research Fellow (ID No: P17102). JSPS is a nonprofit organization.
Rights and permissions
About this article
Cite this article
Sugai, T., Kam, DG., Agathokleous, E. et al. Growth and photosynthetic response of two larches exposed to O3 mixing ratios ranging from preindustrial to near future. Photosynthetica 56, 901–910 (2018). https://doi.org/10.1007/s11099-017-0747-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-017-0747-7