Abstract
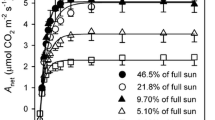
One broad-leaved pioneer tree, Alnus formosana, two broad-leaved understory shrubs, Ardisia crenata and Ardisia cornudentata, and four ferns with different light adaptation capabilities (ranked from high to low, Pyrrosia lingus, Asplenium antiquum, Diplazium donianum, Archangiopteris somai) were used to elucidate the light responses of photosynthetic rate and electron transport rate (ETR). Pot-grown materials received up to 3 levels of light intensity, i.e., 100%, 50% and 10% sunlight. Both gas exchange and chlorophyll (Chl) fluorescence were measured simultaneously by an equipment under constant temperature and 7 levels (0–2,000 μmol m−2 s−1) of photosynthetic photon flux density (PPFD). Plants adapted to-or acclimated to high light always had higher light-saturation point and maximal photosynthetic rate. Even materials had a broad range of photosynthetic capacity [maximal photosynthetic rate ranging from 2 to 23 μmol(CO2) m−2 s−1], the ratio of ETR to gross photosynthetic rate (P G) was close for A. formosana and the 4 fern species when measured under constant temperature, but the PPFD varied. In addition, P. lingus and A. formosana grown under 100% sunlight and measured at different seasonal temperatures (15, 20, 25, and 30°C) showed increased ETR/P G ratio with increasing temperature and could be fitted by first- and second-order equations, respectively. With this equation, estimated and measured P G were closely correlated (r 2 = 0.916 and r 2 = 0.964 for P. lingus and A. formosana, respectively, p<0.001). These equations contain only the 2 easily obtained dynamic indicators, ETR and leaf temperature. Therefore, for some species with near ETR/P G ratio in differential levels of PPFD, these equations could be used to simulate dynamic variation of leaf scale photosynthetic rate under different temperature and PPFD conditions.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- ETR:
-
electron transport rate
- Fv/Fm :
-
potential quantum efficiency of PSII
- g s :
-
stomatal conductance
- P G :
-
gross photosynthetic rate
- P N :
-
net photosynthetic CO2-exchange rate
- PPFD:
-
photosynthetic photon flux density
- PSII:
-
photosystem II
- ΦPSII :
-
PSII efficiency
References
Adams, W.W., III, Zarter, C.R., Ebbert, V., Demmig-Adams, B.: Photoprotective strategies of overwintering evergreens. — BioScience 54: 41–49, 2004.
Aleric, K.M., Kirkman, L.K.: Growth and photosynthetic responses of the federally endangered shrub, Lindera melissifolia (Lauraceae), to varied light environments. — Am. J. Bot. 92: 682–689, 2005.
Asada, K.: The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. — Annu. Rev. Plant Phys. Plant Mol. Biol. 50: 601–639, 1999.
Bazzaz, F.A., Carlson, R.W.: Photosynthetic acclimation to variability in the light environment of early and late successional plants. — Oecologia 54: 313–316, 1982.
Björkman, O., Demmig, B.: Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. — Planta 170: 489–504, 1987.
Blankenship, R.E.: Photosynthesis: The light reactions. — In: Taiz, L., Zeiger, E. (ed.): Plant Physiology 4rd. Pp. 126–158. Sinauer Associates Inc. Publ., Sunderland 2006.
Boardman, N.K.: Comparative photosynthesis of sun and shade plants. — Annu. Rev. Plant Physiol. 28: 355–377, 1977.
Brodribb, T.J., McAdam, S.A.M., Jordan, G.J., Field, T.S.: Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants. — New Phytol. 165: 839–846, 2005.
Cavender-Bares, J., Bazzaz, F.A.: From leaves to ecosystems: Using chlorophyll fluorescence to assess photosynthesis and plant function in ecological studies. — In: Papageorgiou, G.C., Govindjee (ed.): Chlorophyll Fluorescence: A Signature of Photosynthesis. Pp. 737–755. Kluwer Academic Publ., Dordrecht 2004.
Chabot, B.F., Chabot, J.F.: Effects of light and temperature on leaf anatomy and photosynthesis in Fragaria vesca. — Oecologia 26: 363–377, 1977.
Cheng, L., Fuchigami, L.H., Breen, P.J.: The relationship between photosystem II efficiency and quantum yield for CO2 assimilation is not affected by nitrogen content in apple leaves. — J. Exp. Bot. 52: 1865–1872, 2001.
Coopman, R.E., Reyes-Díaz, M., Briceño, V.F., Corcuera, L.J., Cabrera, H.M., Bravo, L.A.: Changes during early development in photosynthetic light acclimation capacity explain the shade to sun transition in Nothofagus nitida. — Tree Physiol. 28: 1561–1571, 2008.
Cornic, G., Briantais, J.M.: Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. — Planta 183: 178–184, 1991.
D’Ambrosio, N., Arena, C., Virzo De Santo, A.: Temperature response of photosynthesis, excitation energy dissipation and alternative electron sinks to carbon assimilation in Beta vulgaris L. — Environ. Exp. Bot. 55: 248–257, 2006.
Dai, Y., Shen, Z., Liu, Y., Wang, L., Hannaway, D., Lu, H.: Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. — Environ. Exp. Bot. 65: 177–182, 2009.
Demmig-Adams, B., Adams, W.W., III: The role of xanthophyll cycle carotenoids in the protection of photosynthesis. — Trends Plant Sci. 1: 21–26, 1996.
Demmig-Adams, B., Adams, W.W., III, Barker, D.H., Logan, B.A., Bowling, D.R., Verhoeven, A.S.: Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. — Physiol. Plant. 98: 253–264, 1996.
Earl, H.J., Tollenaar, M.: Relationship between thylakoid electron transport and photosynthetic CO2 uptake in leaves of three maize (Zea mays L.) hybrids. — Photosynth. Res. 58: 245–257, 1998.
Franco, A., Lüttge, U.: Midday depression in savanna trees: Coordinated adjustments in photochemical efficiency, photorespiration, CO2 assimilation and water use efficiency. — Oecologia 131: 356–365, 2002.
Givnish, T.J.: Adaptation to sun and shade: A whole-plant perspective. — Aust. J. Plant Physiol. 15: 63–92, 1988.
Ghannoum, O., Conroy, J.P., Driscoll, S.P., Paul, M.J., Foyer, C.H., Lawlor, D.W.: Nonstomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. — New Phytol. 159: 599–608, 2003.
Griffin, J.J., Ranney, T.G., Pharr, D.M.: Photosynthesis, chlorophyll fluorescence, and carbohydrate content of Illicium taxa grown under varied irradiance. — J. Amer. Soc. Hort. Sci. 129: 46–53, 2004.
Hall, N.P., Keys, A.J.: Temperature dependence of the enzymic carboxylation and oxygenation of ribulose 1,5-bisphosphate in relation to effects of temperature on photosynthesis. — Plant Physiol. 72: 945–948, 1983.
Haworth, M., Elliott-Kingston, C., McElwain, J.C.: Stomatal control as a driver of plant evolution. — J. Exp. Bot. 62: 2419–2423, 2011.
Hölscher, D., Leuschner, C., Bohman, K., Hagemeier, M., Juhrbandt, J., Tjitrosemito, S.: Leaf gas exchange of trees in old-growth and young secondary forest stands in Sulawesi, Indonesia. — Trees 20: 278–285, 2006.
Huang, J., Boerner, R.E.J., Rebbeck, J.: Ecophysiological responses of two herbaceous species to prescribed burning, alone or in combination with overstory thinning. — Amer. J. Bot. 94: 755–763, 2007.
Kakani, V.G., Surabhi, G.K., Reddy, K.R.: Photosynthesis and fluorescence responses of C4 plant Andropogon gerardii acclimated to temperature and carbon dioxide. — Photosynthetica 46: 420–430, 2008.
Kato, M.C., Hikosaka, K., Hirotsu, N., Makino, A., Hirose, T.: The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. — Plant Cell Physiol. 44: 318–325, 2003.
Krall, J.P., Edwards, G.E.: Quantum yields of photosystem II electron transport and carbon dioxide fixation in C4 plants. — Aust. J. Plant Physiol. 17: 579–588, 1990.
Krall, J.P., Edwards, G.E.: Relationship between photosystem II activity and CO2 fixation in leaves. — Physiol. Plant. 86: 180–187, 1992.
Kubien, D.S., Sage, R.F.: Low-temperature photosynthetic performance of a C4 grass and a co-occurring C3 grass native to high latitudes. — Plant Cell Environ. 27: 907–916, 2004.
Lambers, H., Chapin, F.S., Pons, T.L.: Plant Physiological Ecology. — Springer, New York 1998.
Li, X.P., Björkman, O., Shih, C., Grossman, A.R., Rosenquist, M., Jansson, S., Niyogi, K.K.: A pigment-binding protein essential for regulation of photosynthetic light harvesting. — Nature 403: 391–395, 2000.
Lüttge, U.: Cyanobacterial Tintenstrich communities and their ecology. — Naturwissenschaften 84: 526–534, 1997.
Makino, A., Miyake, C., Yokota, A.: Physiological functions of the water-water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. — Plant Cell Physiol. 43: 1017–1026, 2002.
Maxwell, K., Johnson, G.N.: Chlorophyll fluorescence — a practical guide. — J. Exp. Bot. 51: 659–668, 2000.
Miyake, C., Okamura, M.: Cyclic electron flow within PSII protects PSII from its photoinhibition in thylakoid membranes from spinach chloroplasts. — Plant Cell Physiol. 44: 457–462, 2003.
Oberhuber, W., Dai, Z.Y., Edwards, G.E.: Light dependence of quantum yields of Photosystem II and CO2 fixation in C3 and C4 plants. — Photosynth. Res. 35: 265–274, 1993.
Oberhuber, W., Edwards, G.E.: Temperature dependence of the linkage of quantum yield of photosystem II to CO2 fixation in C4 and C3 plants. — Plant Physiol. 101: 507–512, 1993.
Pearcy, R.W., Sims, D.A.: (1994) Photosynthetic acclimation to changing light environments: Scaling from the leaf to the whole plant. — In: Caldwell, M.M., Pearcy, R.W. (ed.): Exploitation of Environmental Heterogeneity by Plants: Ecophysiological Processes Above- and Belowground. Pp. 145–174. Academic Press, San Diego — NewYork — Boston — London — Sydney -Tokyo — Toronto 1994.
Pérez-Torres, E., Bravo, L.A., Corcuera, L.J., Johnson, G.N.: Is electron transport to oxygen an important mechanism in photoprotection? Contrasting responses from Antarctic vascular plants. — Physiol. Plant. 130: 185–194, 2007.
Peterson, R.B.: Regulation of electron transport in photosystems I and II in C3, C3-C4, and C4 species of Panicum in response to changing irradiance and O2 levels. — Plant Physiol. 105: 349–356, 1994.
Ripley, B.S., Gilbert, M.E., Ibrahim, D.G., Osborne, C.P.: Drought constraints on C4 photosynthesis: Stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. — J. Exp. Bot. 58: 1351–1363, 2007.
Robinson, J.M.: Nitrite photoreduction in vivo is inhibited by oxygen. — Plant Physiol. 92: 862–865, 1990.
Roháček, K., Barták, M.: Technique of the modulated chlorophyll fluorescence: Basic concepts, useful parameters, and some applications. — Photosynthetica 37: 339–363, 1999.
Stuhlfauth, T., Scheuermann, R., Fock, H.P.: Light energy dissipation under water stress conditions: Contribution of reassimilation and evidence for additional processes. — Plant Physiol. 92: 1053–1061, 1990.
Sun, G.C., Zeng, X.P., Liu, X.J., Zhao, P.: Effects of moderate high-temperature stress on photosynthesis in three saplings of the constructive tree species of subtropical forest. — Acta Ecol. Sin. 27: 1283–1290, 2007.
Valladares, F., Pearcy, R.W.: Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. — Plant Cell Environ. 20: 25–36, 1997.
Vavasseur, A., Raghavendra, A.S.: Guard cell metabolism and CO2 sensing. — New Phytol. 165: 665–682, 2005.
Verhoeven, A.S., Adams, W.W., III, Demmig-Adams, B.: The xanthophyll cycle and acclimation of Pinus ponderosa and Malva neglecta to winter stress. — Oecologia 118: 277–287, 1999.
Weng, J.H.: Relationship between allocation of absorbed light energy in PSII and photosynthetic rates of C3 and C4 plants. — Acta Physiol. Plant. 31: 639–647, 2009.
Yu, Q., Zhang, Y.-Q., Liu, Y.-F., Shi, P.-L.: Simulation of the stomatal conductance of winter wheat in response to light temperature and CO2 changes. — Ann. Bot. 93: 435–441, 2004.
Zhang, S.B., Hu, H., Xu, K., Li, Z.R., Yang, Y.P.: Flexible and reversible responses to different irradiance levels during photosynthetic acclimation of Cypripedium guttatum. — J. Plant Physiol. 164: 611–620, 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, S.L., Chen, C.W., Huang, H.W. et al. Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. Photosynthetica 50, 206–214 (2012). https://doi.org/10.1007/s11099-012-0027-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-012-0027-5