Abstract
Background General practice in the UK is experiencing a crisis. Greater multidisciplinary working is a potential solution. The new general practice contract in Scotland encourages this and includes a new pharmacotherapy service to be delivered by General Practice Clinical Pharmacists (GPCPs). Consensus is lacking for the standards of practice for delivery of pharmacotherapy medication reviews (which are polypharmacy and chronic medication reviews) as part of this service. Aim To identify and validate standards of practice for polypharmacy and chronic disease medication (pharmacotherapy level 3) reviews conducted by GPCPs. Method A two-phased mixed-methods consensus methodology was used. Phase 1: An expert group of GPCPs (n = 4) and clinical pharmacist managers (n = 2) responsible for delivering the pharmacotherapy service used a Modified Nominal Group Technique to generate potential standards. Phase 2: Two-round Delphi survey involving GPCPs with ≥ 1 year of experience of working in general practice (n = 159). Results The expert group identified 44 potential standards of practice for polypharmacy and chronic disease reviews. Practicing GPCPs indicated during the Delphi phase that the 44 standards were applicable to practice. The standards of practice covered seven main categories: skills, environment, qualifications, qualities and behaviours, knowledge, process and experience. Conclusion Practicing GPCPs indicated that the standards identified by the expert group are acceptable and valid for current practice and the delivery of polypharmacy and chronic medication reviews. The application of these standards to practice may help GPCPs and general practices to ensure equitable delivery of patient care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Impacts on practice
-
This study identified and validated standards of practice for general practice clinical pharmacists performing polypharmacy and chronic disease medication reviews.
-
The standards covered seven main categories: skills, environment, qualifications, qualities and behaviours, knowledge, process and experience which were applicable to current practice as indicated by practicing general practice clinical pharmacists.
-
Stakeholders may find these standards of use as a platform for further developing and aligning services and professional standards.
Introduction
Healthcare provision around the world is evolving and globally there is a shift in pharmacy multidisciplinary working across Europe, North America and Australasia. There is a greater emphasis on drawing on pharmacists’ knowledge and skills as expert in medicines to optimise care and outcomes for people with polypharmacy and co-morbidities [1,2,3,4]. Pharmacists co-located in general practice clinics, focusing on delivering a range of interventions for patients receiving chronic medications, is becoming more common in many countries [5].
The recruitment crisis of doctors in general practice in the United Kingdom (UK) is well documented [6,7,8]. In part this is due to an aging populations with complex needs, advances in medical care, intensifying workloads, demands and pressures that compromise patient-centred care [8,9,10]. All of this, is driving GPs away from the National Health Service (NHS), their professions, and patients [9, 11]. There is a need to redress these pressures; to enable a better work–life balance to allow GPs to fulfil personal responsibilities and needs [11, 12].
In the UK pharmacists have worked alongside GPs in general practices for over 20 years to improve patient care, outcomes and, more recently, free GP time and capacity [5, 12,13,14,15,16,17]. Consequently, there is a drive to recruit more pharmacists to general practice and help general practice to continue to deliver patient services [18, 19]. The demand for General Practice Clinical Pharmacists (GPCPs) in Scotland has become more urgent since the introduction of the new General Medical Services (GMS) contract in Scotland in 2018 [19]. This contract includes a new three-tiered pharmacotherapy service to be delivered by GPCPs with the aim to optimise patient care and free up GP capacity. Level 1—‘core’ service—includes authorising all prescription requests, immediate discharge letters, outpatient letters, medicine safety reviews and monitoring high risk medicines. Level 2—‘additional advanced’ service- includes medication reviews of ≥ 5 medicines and resolving high risk medicine problems with level 3—‘additional specialist’ service—including polypharmacy reviews and specialist clinics for chronic pain, heart failure, diabetes, etc. [19].
The GMS contract is clear on requirements to provide level 1 and 2 services, however, level 3 service necessitates definition as to how this will be provided to ensure uniformity of care. One of the ways this can be achieved is by developing a set of standards a pharmacist has to meet in order to perform these tasks. A standard is a statement that provides a broad framework and describes how safe and effective care is delivered, hence providing support for pharmacists to improve and shape services [19].
Several sets of standards for pharmacy professionals are available from national organisations within the UK, North America and Australasia. Internationally, in 2012 the International Pharmaceutical Federation (FIP) has published a Global Competency Framework for Pharmacists which provided the basis for national standards set by many pharmacy regulatory bodies around the globe [20,21,22,23]. In the UK, the General Pharmaceutical Council and Royal Pharmaceutical Society published standards for safe and effective pharmaceutical care [24,25,26,27], and the Centre for Postgraduate Pharmacy Education England and NHS Education for Scotland published standards for GPCPs and a GPCP competence framework [28, 29]. All these publications have common themes such as patient-centeredness, good communication, multidisciplinary working, professionalism and speaking up about concerns. While a range of specialist clinical pharmacy service standards and competencies have been defined within the UK and elsewhere, a Canadian study is the only one that considered and developed competencies for pharmacists working in general practice [30,31,32,33,34,35,36,37,38]. This study however did not define standards of practice for the delivery of medication reviews, and hence more research is needed in this area.
Aim
To identify and validate standards of practice for polypharmacy and chronic disease medication reviews (pharmacotherapy level 3) conducted by GPCPs.
Ethics approval
This study received ethical approval from Robert Gordon University School of Pharmacy and Life Science Research Committee, approval number # S225, on 20/12/19.
Method
Design
Two-phase consensus study. Phase 1 applied a Modified Nominal Group Technique (NGT) to generate and achieve consensus on standards of practice for polypharmacy or chronic disease medication review via an expert group. Phase 2, a two-round Delphi questionnaire was applied to gain broader GPCP workforce consensus and validate the standards, in line with CREDES (Conducting and Reporting of Delphi Studies) guidelines (Supplementary file1) [39].
Setting
The UK NHS is a taxpayer funded healthcare system. NHS Greater Glasgow and Clyde (NHSGGC) health board provides healthcare services for a diverse population of 1.2 million people across a varied urban region containing 260 general practices. NHSGGC covers six Health and Social Care Partnerships (HSCPs) and in 2020 employed 159 GPCPs working in general practices.
Phase 1: modified NSGT
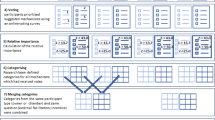
A modified NGT was used, see Fig. 1. An expert group was recruited from experienced GPCPs meeting the following inclusion criteria: experience running regular established clinics for ≥ 4 years and prescribing on a regular basis, and lead clinical pharmacists responsible for service delivery. All, were invited to participate. Co-authors were excluded from participation to reduce bias. A study recruitment email, was sent to all 159 GPCPs via HSCP lead clinical pharmacists in January 2020. A reminder email was sent to encourage participation 2 weeks later. Purposive sampling was employed to ensure that the panel was sufficiently diverse in their experience to ensure validity of results [40]. The NGT was facilitated by the authors (KEP, PF, CJ and HH).
Data generation Consensus methods such as NGT are frequently used for structured idea generation and achieving agreements in healthcare research, especially when little is known on the subject and the opinions of the experts in the field are sought with the benefit of equal participation [41, 42]. A traditional NGT is a structured face-to-face method of generating consensus involving four key stages—idea generation, round robin, clarification of ideas and voting/ranking [43, 44].
This study employed a modified NGT, applying electronic voting/ranking after face-to-face silent idea generation, round robin and clarification of ideas by the expert group (Fig. 1, Stage 1–3). Similar generated standards were then summarised into single standards between round 1 and 2 of ranking. A standardised data collection form for the silent idea generation phase was developed by the lead author (KEP) and the modified NGT process were piloted by KEP and two co-authors (PF and CJ).
Data analysis The standards generated by each participant during the face-to-face meeting populated an electronic Google forms questionnaire and was sent to each participant for ranking; involving two rounds of ranking (Fig. 1). A 5-point Likert-type scale (‘strongly agree’, ‘agree’, ‘neutral’, ‘disagree’ and ‘strongly disagree’) was used. Consensus was defined as ≥ 80% of participants agreeing (‘strongly agree’ or ‘agree’) for a standard to be accepted. Where participants selected ‘strongly disagree’ or ‘disagree’, the—standard was rejected. Standards achieving consensus in round 2 populated the Delphi phase.
Phase 2: Delphi
Delphi questionnaires allow for wide participation and anonymity with two rounds commonly used in the literature, because it is known that > 2 rounds can cause participant fatigue and dropout [44, 45], therefore a two-round Delphi was conducted. The first round was informed by the modified NGT compiled by KEP and piloted by PF and a practicing GPCP.
All experienced GPCPs (≥ 1 year experience in primary care) were invited to participate. This was to allow for a broad and relevant consensus to be achieved. Convenience sampling was employed. Co-authors were excluded from participation to reduce bias. A study recruitment email was sent to all 159 GPCPs via HSCP lead clinical pharmacists for round 1 (March 2020) and round 2 (September 2020—delayed due to COVID-19 pandemic). The email outlined the study, provided a link for the online Webropol questionnaire and requested that participants completed it within a 2 week timeframe. Participants consent to participate was sought prior to completing the questionnaire.
Data analysis The participants rated the presented standards for inclusion using a 5-point Likert scale (see above). Consensus setting varies between NGT and consensus studies, with most common ranges quoted between 70 and 90% [44,45,46,47,48]. Consensus was defined as ≥ 70% of agreement (agree/strongly agree—standard accepted) or disagreement (disagree/strongly disagree—standard rejected). Standards achieving consensus (≥ 70% agree or disagree) were removed before the second round. The results from the second round were analysed using the same method.
Results
Demographics
The NGT expert group consisted of four GPCPs and two lead clinical pharmacists. All participants were female; median age 42 (range 37–53) years old, with a median of 13 years (range 7–20) experience working in general practice. All had experience of running a range of regular clinics (polypharmacy, respiratory, pain, hypertension, cardiology, diabetes) 67% (n = 4) of whom currently delivered patient-facing clinic. All had additional postgraduate qualifications and were independent prescribers.
The Delphi phase captured responses from 59 (37%) and 86 (54%) GPCPs practicing in NHSGGC, for round 1 and 2 respectively (Table 1). Respondent characteristics were similar for round 1 and 2. They were of similar ages, the majority were female, had gained postgraduate qualifications and were prescribers. A similar proportion of GPCPs had experience in running clinics in both groups. In round 2 fewer clinicians reported that they currently ran medication review clinics.
Standards generation
Phase 1: modified NGT
The expert group initially generated 121 standards during the silent generation and round robin phases. Clarification of ideas and the first round of ranking rejected 25 standards: 13 due to duplication; 8 relating to medication review ‘time’; 4 relating to governance (Supplementary file 2). The remaining 96 standards were collapsed/summarised into 47 standards in seven categories (Fig. 1, Table 2) Of the 47 standards: 11 (23%) related to ‘Skills’, 9 (19%) to ‘Environment’, 7 (15%) ‘Qualifications’, 6 (13%) ‘Process’, 6 (13%) ‘Qualities and Behaviours’, 5 (11%) ‘Knowledge’ and 3 (6%) ‘Experience’. Ranking round 2 then resulted in three standards not reaching consensus and being rejected, two of which related to ‘Process’ and one to ‘Environment’. The 44 standards reaching consensus populated the Delphi phase.
Phase 2: Delphi
The first round was completed by 59 (37%) GPCPs, and the second-round by 86 (54%). Consensus was reached during the second-round, all 44 standards proposed by the expert panel being accepted (Table 2).
‘Skills’ was the largest category with 11 standards. These focused on, but were not limited to, taking a holistic patient-centred view when carrying out level 3 reviews, as well as demonstrating the ability to manage complex patients and balance risk of harm and benefits when prescribing and deprescribing. There was also emphasis on signposting and non-pharmacological interventions, the ability to interpret test results for relevant conditions and good time management.
‘Environment’ was the next largest category (n = 8). These standards focused mainly on two areas, firstly, peer support and mentoring for all GPCPs from pharmacists and the wider multidisciplinary team. Secondly, a culture of support within practice ‘to allow full polypharmacy reviews to be conducted’ that incorporated flexibility for repeat appointments within practices and the capacity to conduct reviews in the setting that was ‘most suitable for patients’ e.g. the patients home.
The ‘Qualifications’ category (n = 7), indicated that the GPCPs performing level 3 reviews should be qualified independent prescribers, with up-to-date knowledge and be competent prescribers. Participants demonstrated consensus that GPCPs should have relevant postgraduate qualifications and undertake appropriate additional training such as consultation, communication and relevant clinical examination skills training, suicide prevention training and behaviour skills training.
‘Qualities and Behaviours’ standards (n = 6) focused on GPCPS demonstrating self-awareness, self-motivated and the ability to work independently, but were aware of their own limitations and sought help appropriately. Demonstrating good team working and drawing on individual multidisciplinary team members’ strengths and knowledge was also considered to be important.
‘Knowledge’ standards (n = 5) highlighted the importance of understanding the GPCP role within wider general practice and healthcare team, and the ability to work within different general practice structures and systems. A knowledge of local/national formularies, guidelines and resources to support clinical practice. However, ‘Process’ (n = 4) orientated standards focused on quality improvement, for the GPCP service and personal development through self-reflection and audit practice, and ensuring that good documentation was in place. Lastly, ‘Experience’ standards (n = 3) concentrated on relevant experience that the pharmacists should have in order to deliver effective and efficient service, such as GPCPs having relevant experience in clinical assessment and examination, running clinics and managing caseloads.
Discussion
Key findings
This study identified and validated 44 standards of practice specific to performing polypharmacy and chronic disease medication reviews in general practice. All 44 standards identified by the expert panel reached consensus and were accepted by the Delphi participants, who were experienced GPCPs that are expected to deliver these medication reviews.
The standards covered seven main categorises: skills, environment, qualifications, qualities and behaviours, knowledge, process and experience and concentrated on good communication and patient centeredness with ability to manage complex patients, leadership and team work as well as the ability to work independently. New standards, specific to complex polypharmacy and chronic disease medication reviews in general practice were also identified.
Strengths
To the authors’ knowledge, this is the first study to identify and validate standards of practice and hence standardise polypharmacy and chronic disease medication reviews in general practice. Groups with a maximum of seven participants have been recommended for NGT [41]. The purposive sample of six participants in NGT was small enough to have a close face-to-face discussion, yet large enough to include a broad range of expertise. All the standards generated by the expert group (NGT) were accepted by the GPCPs (Delphi) which adds to the study’s validity. Participants’ demographics were comparable to the recent Scotland wide general practice workforce survey [14], however a higher proportion (83–90%) of respondents were prescribers, which may be explained by further encouragement for independent prescriber qualifications in recent years and the fact that more experienced pharmacists were asked to participate. Another strength was that this study was carried out within single regional health board pharmacy team that have similar management structures, formularies and clinical guidelines.
Limitations
The response rate to Delphi of 37% (n = 59) (round 1) and 54% (n = 86) (round 2) may be considered low but is comparable to previous studies [34, 41]. The study was conducted during COVID pandemic which affected participation. A lower proportion of pharmacists reported current experience of running clinics in round 2 (32%) than round 1 (69%), due to routine clinic cancellations at the height of the pandemic and the second round being conducted during this period. Another potential limitation is that this study did not include non-pharmacy stakeholders i.e. GPs, practice nurses, policy makers, however future studies should consider including such key stakeholders. The NGT employed purposive sampling which resulted in an all-female panel and Delphi employed convenience sampling. These factors may have introduced potential biases, however, the NGT participants had worked in general practice for a median of 13 years, delivering regular clinics and the majority of their standards were accepted by practice GPCPs.
Interpretation
There are a number of implications for policy and practice. The standards generated in our study were similar to those published by the FIP, American College of Clinical Pharmacy and in Canada and Australia, as well as locally in UK and Canadian studies where standards generated for pharmacists working in primary care focussed on patient care in relation to medication related needs [20,21,22,23, 38]. They listed patient-centeredness, effective communication, multidisciplinary collaboration, professionalism and up to date knowledge [21,22,23,24, 38].
Some of the standards identified, however, differ from the above publications, likely due to this study concentrating on identifying standards of practice specifically for conducting polypharmacy and chronic disease medication reviews within general practice. The ‘Qualifications’ and ‘Knowledge’ categories for standards necessitated pharmacists performing medication reviews to be a qualified prescribers, having completed specific clinical examination, assessment and skills courses and suicide prevention training. The independent prescriber qualification allows pharmacists to change patient’s medication at the review and hence ensures the timely and seamless care. Although relevant to UK practice, such skills may not be relevant in other healthcare systems. Additional communication standards in the skills section were also identified, including: the use of motivational techniques, brief interventions and an understanding that sometimes no change is appropriate and ‘planting a seed’ to prepare for the future changes may be just as important.
The Scottish Government’s policy for achieving excellence in pharmaceutical care aligns closely to similar policies in North America and Australasia [1,2,3,4]. These policies focus on the utilisation of pharmacists’ expertise to allow their full integration into multidisciplinary teams to optimise the quality of prescribing and help to deliver the best therapeutic outcomes for patients and their health [1,2,3,4]. The need for advanced pharmacist workforce is clear and there is the need to integrate the developed standards into consultant pharmacist curriculum in order to aid the successful implementation of integrated multidisciplinary working to meet local, national and international visions [1,2,3,4, 49,50,51].
North American and UK studies indicate that there are several factors affecting pharmacist’s abilities to take on new responsibilities and roles in multidisciplinary teams [52,53,54,55,56,57]. In part this is due to a lack of clarity of role, gaps in peer, management and support structures that inhibit optimal multidisciplinary integration, as well as some medical colleagues being anxious about pharmacists’ training and abilities [6, 57]. However, recent evidence demonstrates that the appropriate use of competency frameworks can improve the pharmacist’s performance [58]. Thus, the production of GPCP standards may provide the first step needed to further maximise the performance and effectiveness of the pharmacist clinics, by ensuring uniformity and consistency in delivery.
Further research
Further work will be required to engage key stakeholders from primary care providers, policy makers, educational bodies to members of general practice multidisciplinary teams in order to ensure alignment and maintenance of standards within general practice. Additionally, we need to establish optimal implementation processes for these and other standards by assessing and providing the environmental and professional conditions necessary for evidencing the standards and linkage to nationally approved competency frameworks for advanced pharmacist practice [29, 32]. Furthermore, research is required to explore how to standardise the time resource needed for these reviews, as no consensus was reached on this. Finally, policy makers and professional bodies may find it of use to identify and consider core and specialists standards applicable to generalists and specialists areas of practice.
Conclusion
This study identified the standards of practice for polypharmacy and chronic disease medication reviews in general practice. The identified standards covered seven categories-skills, environment, qualifications, qualities and behaviours, knowledge, process and experience. Similarly, to other standards for pharmacists within the UK and internationally, they concentrated on good communication and patient centeredness with ability to manage complex patients, leadership, team work as well as the ability to work independently. Some new standards, specific to polypharmacy and chronic disease medication reviews in general practice were also identified.
The production of GPCP standards may help to maximise the performance and effectiveness of the pharmacy service, as well as ensuring uniformity and consistency of service delivered. Further research will be required to engage key stakeholders in order to ensure alignment and maintenance of standards within general practice.
References
Canadian Pharmacists Association. Blueprint for pharmacy: designing the future together 2008. https://www.pharmacists.ca/cpha-ca/assets/File/pharmacy-in-canada/blueprint/Blueprint%20Implementation%20Plan_FINAL%20-%20March%202010.pdf. Accessed 3 Mar 2021.
American College of Clinical Pharmacy. A vision of pharmacy’s future roles, responsibilities, and manpower needs in the United States. Pharmacotherapy. 2000;20:991–1020.
Jorgenson D, Penm J, MacKinnon N. Future vision for the pharmacy profession. Can Pharm J (Ott). 2017;150:355–8.
Pharmaceutical Society of Australia Ltd. Pharmacists in 2023: for patients, for our profession, for Australia health system 2019. https://www.psa.org.au/wp-content/uploads/2019/02/Pharmacists-In-2023-digital.pdf. Accessed 5 May 2020.
Tan E, Stewart K, Elliott R. Pharmacist services provided in general practice clinics: a systematic review and meta-analysis. Res Soc Adm Pharm. 2014;10:608–22.
Nelson P, Bradley F, Martindale A, et al. Skill-mix change in general practice: a qualitative comparison of three ‘new’ non-medical roles in English primary care. Br J Gen Pract. 2019;69:489–98.
Baird B. Is general practice in crisis? Big election questions. The Kings Fund 2017. https://www.kingsfund.org.uk/publications/articles/big-election-questions-gp-crisis. Accessed 8 July 2021.
Maskrey M, Johnson C, Cormack J, et al. Releasing GP capacity with pharmacy prescribing support and new ways of working: a prospective observational cohort study. B J Gen Pract. 2018;68:735–42.
Doran N, Fox F, Rodham K, et al. Lost to the NHS: a mixed methods study of why GPs leave practice early in England. Br J Gen Pract. 2016;66:128–35.
Lachish S, Svirko E, Goldacre MJ, et al. Factors associated with less than-full-time working in medical practice: results of surveys of five cohorts of UK doctors, 10 years after graduation. Hum Resour Health. 2016;14:62.
Norman R, Hall JP. The desire and capability of Australian general practitioners to change their working hours. Med J Aust. 2014;200:399–402.
Lowrie R, Lloyd S, Mcconnachie A, et al. A cluster randomised controlled trial of a pharmacist-led collaborative intervention to improve statin prescribing and attainment of cholesterol targets in primary care. PLoS ONE. 2014;9:e113370.
Johnson C, Thomson A. Prescribing support pharmacists support appropriate benzodiazepine and Z-drug reduction 2008/2009—experiences from North Glasgow. Clin Pharm. 2010;3(Supp1):S5-6.
Stewart D, MacLure K, Newham R, et al. A cross-section survey of pharmacy workforce in general practice in Scotland. Fam Pract. 2019;37:206–12.
Hunt V, Anderson D, Lowrie R, et al. A non-randomised controlled pilot study of clinical pharmacist collaborative intervention for community dwelling patients with COPD. NPJ Prim Care Respir Med. 2018;28:38.
Mackie CA, Lawson DH, Campbell A, et al. A randomised controlled trial of medication review in patients receiving polypharmacy in a general practice setting. Pharm J. 1999;263:R7.
Scottish Government. Primary care investment: three-year funding to support front-line community care. 2015. https://www.wired-gov.net/wg/news.nsf/articles/Primary+care+investment+25062015171200. Accessed 9 July 2021.
NHS England. More than 400 pharmacists to be recruited to GP surgeries by next year. 2015. https://www.england.nhs.uk/2015/11/pharmacists-recruited/. Accessed 1 May 2021.
The Scottish Government. The 2018 general medical services contract in Scotland. Edinburgh. 2017. https://www.bma.org.uk/what-we-do/committees/general-practitioners-committee/scottish-general-practitioners-committee. Accessed 1 May 2021.
International Pharmaceutical Federation. Pharmacy education taskforce. A global competency framework 2012. https://www.fip.org/files/fip/PharmacyEducation/GbCF_v1.pdf. Accessed 1 May 2021.
Pharmaceutical Society of Australia. National competency standards framework for pharmacists in Australia 2016. https://www.psa.org.au/wp-content/uploads/2018/06/National-Competency-Standards-Framework-for-Pharmacists-in-Australia-2016-PDF-2mb.pdf. Accessed 7 July 2021.
Pharmaceutical Society of Australia. Professional practice standards. 2017. https://my.psa.org.au/s/article/Professional-Practice-Standards. Accessed 7 July 2021.
National Association of Pharmacy Regulatory Authorities. Professional competencies for Canadian pharmacists at entry to practice 2014. https://napra.ca/pharmacists/professional-competencies-canadian-pharmacists-entry-practice-2014. Accessed 20 July 2021.
General Pharmaceutical Council. Standards for pharmacy professionals 2017. https://www.pharmacyregulation.org/sites/default/files/standards_for_pharmacy_professionals_may_2017_0.pdf. Accessed 10 July 2021.
Royal Pharmaceutical Society. A competency framework for all prescribers 2016. https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Professional%20standards/Prescribing%20competency%20framework/prescribing-competency-framework.pdf?ver=2019-02-13-163215-030. Accessed 10 July 2021.
Royal Pharmaceutical Society. The RPS Advanced Pharmacy framework (APF) 2013. https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Frameworks/RPS%20Advanced%20Pharmacy%20Framework.pdf. Accessed 1 May 2021.
Pharmaceutical Society of Northern Ireland. THE CODE Professional standards of conduct, ethics and performance for pharmacists in Northern Ireland 2016. https://www.psni.org.uk/wp-content/uploads/2012/09/22504-PSNI-Code-of-Practice-Book-final.pdf. Accessed 1 May 2021.
Centre for Pharmacy Post Graduate Education. Developing clinical pharmacists in general practice. The national learning pathway 2016. https://www.cppe.ac.uk/wizard/files/developing_career/cppe%20hee%20general%20practice%20pharmacist%20learning%20pathway%203rd%20edition.pdf. Accessed 1 May 2021.
National Education for Scotland. General practice clinical pharmacist competency & capability framework—a user’s manual 2016. https://nesvleprdstore.blob.core.windows.net/nesndpvlecmsprdblob/43cffd66-9ea5-46df-b806-11886ecdb1ef_2016-09-02%20Competency%20framework%20for%20pharmacists%20final.pdf?sv=2018-03-28&sr=b&sig=0ZRyEUq7Z6l3cic%2BGKYrFlIO8%2BO4n48Z94VxL2GRzfE%3D&st=2022-02-11T22%3A13%3A06Z&se=2022-02-11T23%3A18%3A06Z&sp=r. Accessed 1 May 2021. NES turas portal access required.
Forsyth P, Warren A, Thomson C, et al. A competency framework for clinical pharmacists and heart failure. Int J Pharm Pract. 2018;27:424–35.
Carrington C, Weir J, Smith P. The development of a competency framework for pharmacists providing cancer services. J Oncol Pharm Pract. 2011;17:168–78.
Murphy L, Chang F, Dattani S, et al. A pharmacist framework for implementation of the Canadian guideline for opioids for chronic non-cancer pain. Can Pharm J (Ott). 2019;152:35–44.
Raymond C, Wazny L, Sood A. Standards of clinical practice for renal pharmacists. Can J Hosp Pharm. 2013;66:369–74.
Boullata J, Holcombe B, Gervasio J, et al. Standardized competencies for parenteral nutrition order review and parenteral nutrition preparation, including compounding. The ASPEN Model. Nutr Clin Pract. 2016;31:548–55.
Mackler E, Segal E, Muluneh B, et al. 2018 Haematology/Oncology Pharmacist Association best practice for management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15:e346–55.
Rollins C, Durfee S, Holcombe B, et al. Standards of practice for nutrition support pharmacists American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) task force for revision of nutrition support pharmacist standards: 2008. Nutr Clin Pract. 2008;23:189–94.
Tucker A, Ybarra J, Bingham A, et al. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) standards of practice for nutrition support pharmacists. Nutr Clin Pract. 2015;30:139–46.
Kennie-Kaulbach N, Farrell B, Ward N, et al. Pharmacist provision of primary health care: a modified Delphi validation of pharmacists’ competencies. BMC Fam Pract. 2012;13:1–9.
Junger S, Payne SA, Brine J, et al. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. J Palliat Med. 2017;31:684–706.
Palinkas L, Horwitz S, Green C, et al. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42:533–44.
McMillan S, King M, Tully M. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38:655–626.
Jones J, Hunter D. Consensus methods for medical and health services research. Br Med J. 1995;311:376–80.
Perry J, Linsley S. The use of nominal group technique as an evaluative tool in the teaching and summative assessment of the inter-personal skills of student mental health nurses. Nurse Educ Today. 2006;26:346–53.
Centers for Disease Control and Prevention. Evaluation Briefs No. 7. Gaining consensus among stakeholders through the nominal group technique. https://www.cdc.gov/healthyyouth/evaluation/pdf/brief7.pdf. Accessed 9 July 2021.
Boulkedid R, Abdoul H, Loustau M, et al. Using and reporting the Delphi method for selecting healthcare quality indicators: a systemic review. PLoS ONE. 2011;6:e20476.
Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67:401–9.
Hsu CC, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12:1–8.
Foth T, Efstathiou N, Vanderspank-Wright B, et al. The use of Delphi and Nominal Group Technique in nursing education: a review. Int J Nurs Stud. 2016;60:112–20.
Neville L, Swift J. Measuring the impact of the advanced practitioner role: a practical approach. J Nurs Manag. 2012;20:382–9.
Bates I, Bader L, Galbraith K. A global survey on trends in advanced practice and specialisation in the pharmacy workforce. Int J Pharm Pract. 2020;28:173–81.
Lawler J, MacIaine K, Leary A. Workforce experience of the implementation of an advanced clinical practice framework in England: a mixed methods evaluation. Hum Resour Health. 2020;18:96.
Rosenthal M, Austin Z, Tsuyuki RT. Are pharmacists the ultimate barrier to pharmacy practice change? CPJ. 2010;143:37–42.
Fisher J, Kinnear M, Reid F, et al. What supports hospital pharmacist prescribing in Scotland?—a mixed methods, exploratory sequential study. Res Social Adm Pharm. 2018;14:488–97.
Zhou M, Desborough J, Parkinson A, et al. Barriers to pharmacist prescribing: a scoping review comparing the UK, New Zealand, Canadian and Australian experiences. Int J Pharm Pract. 2019;27:479–89.
Rosenthal MM, Austin Z, Farrell J, et al. Overcoming our nature and nurture. CPJ. 2017;150:5–7.
Maddox C, Halsall D, Hall J, et al. Factors influencing nurse and pharmacist willingness to take or not take responsibility for non-medical prescribing. Res Social Adm Pharm. 2016;12:41–55.
Rokib T. Pharmacist led care in General Practice (PLAGE) Study. Pharmacy Research UK. https://pharmacyresearchuk.org/wp-content/uploads/2017/01/Pharmacist-led-care-in-General-Practice.pdf. Accessed 8 Mar 2021.
Udoh A, Bruno-Tomé A, Ketut Ernawati D, et al. The effectiveness and impact on performance of pharmacy-related competency development frameworks: a systematic review and meta-analysis. Res Social Adm Pharm. 2021;17:1697–718.
Acknowledgements
We would like thank all the primary care pharmacists who participated in this study.
Funding
No funding was received for conducting this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Earle-Payne, K., Forsyth, P., Johnson, C.F. et al. The standards of practice for delivery of polypharmacy and chronic disease medication reviews by general practice clinical pharmacists: a consensus study. Int J Clin Pharm 44, 663–672 (2022). https://doi.org/10.1007/s11096-022-01387-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01387-7