Abstract
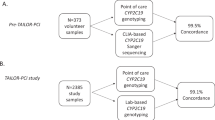
Background A quick CYP2C19*2 genotyping assay can be useful in personalised antiplatelet-therapy. Objective To apply a rapid point-of-care (POC) CYP2C19*2 genotyping assay for personalisation of antiplatelet therapy in patients undergoing percutaneous coronary intervention (PCI) and to compare this POC assay to two laboratory-based CYP2C19*2 genotyping assays. Setting Cardiac Catheterisation Suite and Molecular Diagnostics Unit in a general hospital. Methods A buccal sample was collected for POC CYP2C19*2 genotyping with the Spartan™ RX system (Spartan Bioscience). A whole blood sample was collected from the same patients for laboratory-based CYP2C19*2 genotyping with a TaqMan® allelic discrimination assay (Life Technologies) using real-time quantitative PCR and with the GenID® reverse dot-blot hybridisation assay (Autoimmun Diagnostika GmbH). Each patient was genotyped as a non-carrier of CYP2C19*2 (*1/*1), a carrier of one CYP2C19*2 allele (*1/*2), or a carrier of two CYP2C19*2 alleles (*2/*2). Genotyping, interpretation and communication of genotype results (*1/*2, *2/*2) to the consultant cardiologist was undertaken by a clinical pharmacist researcher. Quantitative and qualitative comparison between the three assays was carried out. Main outcome measures Application of a rapid POC CYP2C19*2 genotyping assay for antiplatelet therapy individualisation; comparison of the POC CYP2C19*2 genotyping assay to two laboratory-based assays. Results The total sample consisted of 34 Caucasian patients. With the POC assay, 21 patients were genotyped as non-carriers of CYP2C19*2, 12 patients as carriers of one CYP2C19*2 allele and one patient as a carrier of two CYP2C19*2 alleles. With both laboratory-based assays, the same 21 patients were genotyped as non-carriers of CYP2C19*2, however 13 patients were genotyped as carriers of one CYP2C19*2 allele and no patients were genotyped as carriers of two CYP2C19*2 alleles. Agreement in genotype results was 97 % (κ = 0.939) between the POC assay and both laboratory-based assays and 100 % (κ = 1.000) between the two laboratory-based assays. Conclusion Compared to both laboratory-based genotyping assays, the POC assay is accurate and reliable, provides rapid results, can process single samples, is portable and more operator-friendly, however the tests are more expensive.
Similar content being viewed by others
References
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619.
Knauer MJ, Diamandis EP, Hulot JS, Kim RB, So DY. Clopidogrel and CYP2C19: pharmacogenetic testing ready for clinical prime time? Clin Chem. 2015;61(10):1235–40.
Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genom. 2010;20(7):463–5.
Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38(1):92–9.
Geisler T, Schaeffeler E, Gawaz M, Schwab M. Genetic variation of platelet function and pharmacology: an update of current knowledge. Thromb Haemost. 2013;110(5):876–7.
Scott SA, Sangkuhl K, Shuldiner AR, Hulot JS, Thorn CF, Altman RB, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet Genom. 2012;22(2):159–65.
Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317–23.
Yang Y, Lewis JP, Hulot JS, Scott SA. The pharmacogenetic control of antiplatelet response: candidate genes and CYP2C19. Expert Opin Drug Metab Toxicol. 2015;11(10):1–19.
Food and Drugs Administration (FDA). FDA announces new boxed warning on Plavix [Online]. USA: FDA News Release; 12 March 2010 (cited 2 Sept 2015). www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm204253.htm.
Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, et al. Cytochrome P450 2C19 681G > A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51(20):1925–34.
Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–17.
Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75.
Nestorovska AK, Cvetkovska AD, Suturkova L. Association between CYP2C19*2 variant and clinical outcome in clopidogrel treated patients from Republic of Macedonia. Maced Pharm Bull. 2010;56(1,2):37–44.
Campo G, Parrinello G, Ferraresi P, Lunghi B, Tebaldi M, Miccoli M, et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol. 2011;57(25):2474–83.
Pettersen AA, Arnesen H, Opstad TB, Seljeflot I. The influence of CYP2C19*2 polymorphism on platelet function testing during single antiplatelet treatment with clopidogrel. Thromb J. 2011;9:4.
Tello-Montoliu A, Jover E, Marín F, Bernal A, Lozano ML, Sánchez-Vega B, et al. Influence of CYP 2C19 polymorphisms in platelet reactivity and prognosis in an unselected population of non ST elevation acute coronary syndrome. Rev Esp Cardiol (Engl Ed). 2012;65(3):219–26.
Nyírő G, Inczédy-Farkas G, Reményi V, Gál A, Pál Z, Molnár MJ. The effect of the CYP2C19*2 polymorphism on stroke care. Acta Physiol Hung. 2012;99(1):33–9.
Siller-Matula JM, Delle-Karth G, Lang IM, Neunteufl T, Kozinski M, Kubica J, et al. Phenotyping vs. genotyping for prediction of clopidogrel efficacy and safety: the PEGASUS-PCI study. J Thromb Haemost. 2012;10(4):529–42.
Carlquist JF, Knight S, Horne BD, Huntinghouse JA, Rollo JS, Muhlestein JB, et al. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. Thromb Haemost. 2013;109(4):744–54.
Jeong YH, Tantry US, Kim IS, Koh JS, Kwon TJ, Park Y, et al. Effect of CYP2C19*2 and *3 loss-of-function alleles on platelet reactivity and adverse clinical events in East Asian acute myocardial infarction survivors treated with clopidogrel and aspirin. Circ Cardiovasc Interv. 2011;4(6):585–94.
Gold B. Origin and utility of the reverse dot-blot. Expert Rev Mol Diagn. 2003;3(2):143–52.
Yildirim R, Gündoğdu M, Kurnaz F, Yildirim A, Aksoy H, Erdem F, et al. CYP2C9 gene polymorphisms and warfarin dose requirement: a single-center experience in Turkey. Turk J Med Sci. 2012;42(6):981–6.
Spizz G, Chen Z, Li P, McGuire IC, Klimkiewicz P, Zysling D, et al. Determination of genotypes using a fully automated molecular detection system. Arch Pathol Lab Med. 2015;139(6):805–11.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. (Academic Research Consortium). Clinical end points in coronary stent trials: a case for standardised definitions. Circulation. 2007;115(17):2344–51.
Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, et al. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol. 2009;103(6):806–11.
Swen JJ, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee AH, Mulder H, et al. Pharmacogenetics: from bench to byte—an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662–73.
Roberts JD, Wells GA, Le May MR, Labinaz M, Glover C, Froeschl M, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379(9827):1705–11.
Stimpfle F, Karathanos A, Droppa M, Metzger J, Rath D, Müller K, et al. Impact of point-of-care testing for CYP 2C19 on platelet inhibition in patients with acute coronary syndrome and early dual antiplatelet therapy in the emergency setting. Thromb Res. 2014;134(1):105–10.
So DY, Wells GA, McPherson R, Labinaz M, Le May MR, Glover C et al. A prospective randomized evaluation of a pharmacogenomic approach to antiplatelet therapy among patients with ST-elevation myocardial infarction: the RAPID STEMI study. Pharmacogenomics J. 2016;16(1):71–8.
Mason G, Wirth F, Cignarella A, Xuereb RG, Azzopardi LM. Antiplatelet and anticoagulant therapy for non-ST-elevation acute coronary syndromes in a general hospital. Online J Clin Audits. 2014;6(3):7–16.
Jiang M, You JH. Review of pharmacoeconomic evaluation of genotype-guided antiplatelet therapy. Expert Opin Pharmacother. 2015;16(5):771–9.
Reese ES, Daniel Mullins C, Beitelshees AL, Onukwugha E. Cost-effectiveness of cytochrome P450 2C19 genotype screening for selection of antiplatelet therapy with clopidogrel or prasugrel. Pharmacotherapy. 2012;32(4):323–32.
Panattoni L, Brown PM, Te Ao B, Webster M, Gladding P. The cost effectiveness of genetic testing for CYP2C19 variants to guide thienopyridine treatment in patients with acute coronary syndromes: a New Zealand evaluation. Pharmacoeconomics. 2012;30(11):1067–84.
Lala A, Berger JS, Sharma G, Hochman JS, Scott Braithwaite R, Ladapo JA. Genetic testing in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cost-effectiveness analysis. J Thromb Haemost. 2013;11(1):81–91.
Kazi DS, Garber AM, Shah RU, Dudley RA, Mell MW, Rhee C, et al. Cost-effectiveness of genotype-guided and dual antiplatelet therapies in acute coronary syndrome. Ann Intern Med. 2014;160(4):221–32.
Johnson SG, Gruntowicz D, Chua T, Morlock RJ. Financial analysis of CYP2C19 genotyping in patients receiving dual antiplatelet therapy following acute coronary syndrome and percutaneous coronary intervention. J Manag Care Spec Pharm. 2015;21(7):552–7.
American Society of Health-System Pharmacists (ASHP). ASHP statement on the pharmacist’s role in clinical pharmacogenomics. Am J Health Syst Pharm. 2015;72(7):579–81.
Johnson JA, Elsey AR, Clare-Salzler MJ, Nessl D, Conlon M, Nelson DR. Institutional profile: University of Florida and Shands Hospital Personalized Medicine Program: clinical implementation of pharmacogenetics. Pharmacogenomics. 2013;14(7):723–6.
Shuldiner AR, Palmer K, Pakyz RE, Alestock TD, Maloney KA, O’Neill C, et al. Implementation of pharmacogenetics: the University of Maryland Personalized Antiplatelet Pharmacogenetics Program. Am J Med Genet C Semin Med Genet. 2014;166C(1):76–84.
Ferreri SP, Greco AJ, Michaels NM, O’Connor SK, Chater RW, Viera AJ, et al. Implementation of a pharmacogenomics service in a community pharmacy. J Am Pharm Assoc. 2014;54(2):172–80.
Kisor DF, Bright DR, Conaway M, Bouts BA, Gerschutz GP. Pharmacogenetics in the community pharmacy: thienopyridine selection post-coronary artery stent placement. J Pharm Pract. 2014;27(4):416–9.
Acknowledgments
This research was carried out in collaboration with all consultant cardiologists and staff at the Cardiology Department at Mater Dei Hospital. The authors would like to thank the Pharmacogenetics Laboratory of the Institute of Biochemistry in the Faculty of Medicine at the University of Ljubljana, Slovenia, and the Department of Clinical Pharmacy and Toxicology at the Leiden University Medical Center, The Netherlands, for providing the positive controls used in the real-time PCR genotyping. The authors would also like to thank Professor Liberato Camilleri, Head of the Department of Statistics and Operations Research in the Faculty of Science at the University of Malta, for his assistance with the statistical analysis.
Funding
This research was financially supported by the University of Malta’s Faculty of Medicine and Surgery Dean’s Initiative, the University of Malta Research Grant on Point-of-Care Testing, Technoline Ltd., Scientech Ltd., E.J. Busuttil Ltd., Malta Heart Foundation, AID Diagnostika GmbH, Orme Scientific Ltd. and LEVO Laboratory Services Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest to disclose.
Rights and permissions
About this article
Cite this article
Wirth, F., Zahra, G., Xuereb, R.G. et al. Comparison of a rapid point-of-care and two laboratory-based CYP2C19*2 genotyping assays for personalisation of antiplatelet therapy. Int J Clin Pharm 38, 414–420 (2016). https://doi.org/10.1007/s11096-016-0269-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-016-0269-6