Abstract
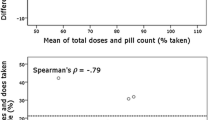
Objective To evaluate the validity of patient self-reported adherence, and to find the optimal length of recall period which best reflects the long-term adherence pattern of the patient. Setting Patients were recruited from a general practitioner’s practice in a Hungarian town. Method In this prospective study 30 patients, who had already been on antihypertensive treatment, were involved. The study was designed to monitor one antihypertensive medication per patient for 3 months. Patients received a 3-month supply of one antihypertensive medication in an electronic Medication Event Monitoring System (MEMS). At the end of the study period patients completed a structured questionnaire regarding their medication taking behavior during the last 7, 14 and 30 days. The results measured with MEMS were considered as the reference value, and other measures were compared using the Bland–Altman method. Main outcome measures self-reported adherence, length of recall period, taking adherence and timing adherence measured by MEMS. Results Of the 30 patients included, 29 patients (13 males and 16 females) completed the study. The mean age of the patients was 60.6 years, ranging between 36 and 86 years. Patients were monitored for an average of 89 days (ranging between 49 and 106 days). Fifteen patients were on once daily, 9 patients were on twice daily, and 5 patients were on 3 times daily dosing schedule. The total expected number of medication taking events was 4,281. The MEMS caps recorded a total of 4,071 openings, which showed only a 3.56% deviation from the pill counts of the remaining tablets. The overall taking adherence was 95.1%, timing adherence was 75.2%. Patients’ adherence report using a visual analog scale and reporting the number of missed doses became more accurate as the length of the recall period increased. Increased number of chronically taken medications was associated with better adherence. Increased dosing frequency of the observed antihypertensive medication resulted in decreased adherence. Conclusion The results showed that the length of the recall period influences the accuracy of self-reported adherence. Patients seem to be able to report more precisely their medication taking behavior regarding a 30 day period than a 7 day period.
Similar content being viewed by others
References
World Health Organization. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003. ISBN 9241545992.
McWhinney DB. Reducing the human and economic costs of drug therapy complications: responding to the medication safety issue. Cardinal Health, Inc., Version 5.0, Available online at: http://www.cardinal.com/patientsafety/medication/article3.pdf. Accessed September 28, 2010.
Corrao G, Parodi A, Nicotra F, Zambon A, Merlino L, Cesana G, et al. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29(3):610–8.
Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–605.
Morgado M, Rolo S, Castelo-Branco M. Pharmacist intervention program to enhance hypertension control: a randomised controlled trial. Int J Clin Pharm. 2011;33(1):132–40.
Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336(7653):1114–7.
McElnay JC, McCallion CR, Al-Deagi F, Scott M. Self-reported medication non-compliance in the elderly. Eur J Clin Pharmacol. 1997;53:171–8.
Shi L, Liu J, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices. Pharmacoeconomics. 2010;28(12):1097–107.
Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care. 2004;42(7):649–52.
Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life Outcomes. 2010;8:99.
Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. 1999;37(2):113–24.
Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–45.
Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12(1):86–94.
Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–77.
Oyugi JH, Byakika-Tusiime J, Charlebois ED, Kityo C, Mugerwa R, Mugyenyi P, Bangsberg DR. Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource-limited setting. J Acquir Immune Defic Syndr. 2004;36(5):1100–2.
Schroeder K, Fahey T, Hay DA, Montgomery A, Peters JT. Adherence to antihypertensive medication assessed by self-report was associated with electronic monitoring compliance. J Clin Epidemiol. 2006;59:650–1.
Hungarian National Health Fund Administration. National drug utilization data. Available at: http://www.oep.hu. Accessed October 5, 2010.
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74.
Steeg N, Sielk M, Pentzek M, Bakx C, Altiner A. Drug-adherence questionnaires not valid for patients taking blood-pressure lowering drugs in a primary health care setting. J Eval Clin Pract. 2009;15:468–72.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother. 2004;38(9):1363–8.
Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27:2149–53.
Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–10.
Zeller A, Schroeder K, Peters TJ. Electronic pillboxes (MEMS) to assess the relationship between medication adherence and blood pressure control in primary care. Scand J Prim Health. 2007;25:202–7.
Zeller A, Schroeder K, Peters TJ. An adherence self-report questionnaire facilitated the differentiation between nonadherence and nonresponse to antihypertensive treatment. J Clin Epidemiol. 2008;61:282–8.
Vitolins MZ, Rand CS, Rapp SR, Ribisl PM, Sevick MA. Measuring adherence to behavioral and medical interventions. Control Clin Trials. 2000;21:188S–94S.
Jank S, Bertsche T, Schellberg D, Herzog W, Haefeli WE. The A14-scale: development and evaluation of a questionnaire for assessment of adherence and individual barriers. Pharm World Sci. 2009;31(4):426–31.
Grant RW, Devita NG, Singer DE, Meigs JB. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26:1408–12.
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–310.
Lau HS, Beuning KS, Postma-Lim E, Klein-Beernink L, de Boer A, Porsius AJ. Non-compliance in elderly people: evaluation of risk factors by longitudinal data analysis. Pharm World Sci. 1996;18(2):63–8.
Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22–33.
Imai Y, Ohkubo T, Kikuya M, Hashimoto J. Practical aspect of monitoring hypertension based on self-measured blood pressure at home. Intern Med. 2004;43(9):771–8.
Tislér A, Dunai A, Keszei A, Fekete B, Othmane Tel H, Torzsa P, et al. Primary-care physicians’ views about the use of home/self blood pressure monitoring: nationwide survey in Hungary. J Hypertens. 2006;24(9):1729–35.
Acknowledgments
None.
Funding
The study did not receive any external funding; it was solely financed by the Department of Clinical Pharmacy University of Szeged.
Conflicts of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doró, P., Benkő, R., Czakó, A. et al. Optimal recall period in assessing the adherence to antihypertensive therapy: a pilot study. Int J Clin Pharm 33, 690–695 (2011). https://doi.org/10.1007/s11096-011-9529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-011-9529-7