Abstract
Purpose
The purpose of the study is to present a mathematical model capable of describing drug particle dissolution in 3-dimensional (3D) space, and to provide experimental model verification. Through this study, we also aim to elaborate limitations of the classic, 1D-based Nernst-Brunner formalism in dissolution modeling.
Methods
The 3D dissolution model was derived by treating the dissolution of a spherical particle as a diffusion-driven process, and by solving Fick’s 2nd law of diffusion in spherical coordinates using numerical methods. The resulting model was experimentally verified through analyzing the dissolution behavior of single succinic acid particles in un-stirred water droplet under polarized light microscopy, in combination with image segmentation techniques.
Results
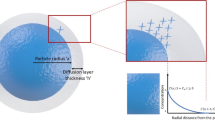
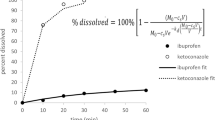
A set of working equations was developed to describe drug particle dissolution in 3D space. The predicted dissolution time and profile are in good agreement with the experimental results. The model clearly shows that the concentration gradient within the diffusion layer, in realistic 3D condition, must not be a constant value as implicated in the Nernst-Brunner formalism. The actual concentration profile is a hyperbola, and the concentration gradient at the surface of the particle can be significantly higher than the classic 1D-based dissolution model.
Conclusion
The study demonstrates that the classic, 1D-based dissolution models may lead to significant under-estimation of drug dissolution rates. In contrast, modeling dissolution in 3D space yields more reliable results. This study merits further development of comprehensive 3D drug dissolution models, by considering polydispersed particle ensemble and imposing the changes of diffusion layer thickness during dissolution.










Similar content being viewed by others
References
Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. 2006;321(1–2):1–11.
Siepmann J, Siepmann F. Mathematical modeling of drug dissolution. Int J Pharm. 2013;453(1):12–24.
Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19(12):930–4.
Brunner E. Reaktionsgeschwindigkeit in heterogenen Systemen. Z Phys Chem. 1904;47(1):56–102.
Nernst W. Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Z Phys Chem. 1904;47(1):52–5.
Danckwerts P. Significance of liquid-film coefficients in gas absorption. Ind Eng Chem. 1951;43(6):1460–7.
Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50(10):874–5.
Hintz RJ, Johnson KC. The effect of particle size distribution on dissolution rate and oral absorption. Int J Pharm. 1989;51(1):9–17.
Lu AT, Frisella ME, Johnson KC. Dissolution modeling: factors affecting the dissolution rates of polydisperse powders. Pharm Res. 1993;10(9):1308–14.
Sugano K. Introduction to computational oral absorption simulation. Expert Opinion On Drug Metabolism & tToxicology. 2009;5(3):259–93.
Ranz W, Marshall WJ. Evaporation from drops Part I. Chem Eng Prog. 1952;48:141–6.
Martin A, Bustamante P, Chun A. Diffusion and Dissolution. In: Physical Pharmacy. Philadelphia, PA, USA: Lea & Febiger; 1993. p. 324–361.
Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory. Journal of Pharmaceutical Sciences. 1999;88(7):731–8.
Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 2. Evaluation of experimental data. Journal of Pharmaceutical Sciences. 2002;91(2):534–42.
Hixson A, Crowell J. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;23(8):923–31.
Hixson A, Crowell J. Dependence of reaction velocity upon surface and agitation. II. Experimental procedure in study of surface. Ind Eng Chem. 1931;23:1002–9.
Crank J. The Mathematics of Diffusion. Bristol, UK: Oxford University Press; 1975.
Korenev BG. Bessel Functions and Their Applications. Boca Raton, FL, USA: CRC Press; 2002.
Boas ML. Mathematical Methods in the Physical Sciences. In. Hoboken. NJ, USA: John Wiley & Sons; 2006. p. 340–89.
Shampine LF, Reichelt MW. The matlab ode suite. SIAM J Sci Comput. 1997;18(1):1–22.
Shorten RB. Approximate Analytic Solution of the One Phase Stefan Problem for the Sphere. In: IUTAM Symposium on Recent Advances in Moving Boundary Problems in Mechanics. Cham, Switzerland: Springer International Publishing; 2019. p. 185–201.
Wu B, McCue SW, Tillman P, Hill JM. Single phase limit for melting nanoparticles. Appl Math Model. 2009;33(5):2349–67.
Hu Y-H, Chen Z-G, Yang W-G, Shi Y, Sun H-L, Li Y-L. Solubility of succinic acid in ethanol plus water systems from 278.15 K to 333.15 K. Journal of Solution Chemistry. 2013;42(1):102–10.
Kim H. Diffusion studies of the systems water-succinic acid-urea and water-succinic acid at 25° C: The effect of complex formation on the diffusion coefficients of the ternary system. J Solution Chem. 1974;3(4):271–87.
Sugano K. Biopharmaceutics Modeling and Simulations: Theory, Practice, Methods, and Applications. Hoboken, New Jersey: John Wiley & Sons; 2012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interests/Competing Interests
The authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
So, C., Chiang, PC. & Mao, C. Modeling Drug Dissolution in 3-Dimensional Space. Pharm Res 39, 907–917 (2022). https://doi.org/10.1007/s11095-022-03270-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03270-6