Abstract
Purpose
Hydrogen/deuterium (H/D) exchange over a range of temperatures suggests a protein structural/mobility transition in the solid state below the system glass transition temperature (Tg). The purpose of this study was to determine whether solid-state protein stability correlates with the difference between storage temperature and apparent Td where an abrupt change in mobility occurs, or alternatively, the extent of H/D exchange at a single temperature correlates directly to protein stability in lyophilized solids.
Methods
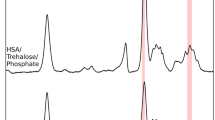
Solid-state H/D exchange was monitored by FTIR spectroscopy to study the extent of exchange and the apparent transition temperature in both pure recombinant human serum albumin (rHSA) and rHSA formulated with sucrose or trehalose. H/D exchange of freeze-dried formulations at 11% RH and temperatures from 30 to 80°C was monitored. Protein stability against aggregation at 40°C/11% RH for 6 months was assessed by size exclusion chromatography (SEC).
Results
Both sucrose and trehalose showed equivalent protection of protein secondary structure by FTIR. The rHSA:sucrose formulation showed superior long-term stability at 40°C by SEC over the trehalose formulation, but the apparent Td determined from H/D exchange was much higher in the trehalose formulation. Instead, the extent of H/D exchange (X∞) was lower in the sucrose formulation at the temperature of the stability studies (40°C) than found for the trehalose formulation, which was consistent with better stability in the sucrose formulation.
Conclusions
While apparent Td did not correlate with protein stability for rHSA, the extent of H/D exchange, X∞, did.







Similar content being viewed by others
Change history
23 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11095-021-03016-w
References
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42.
Hill JJ, Shalaev EY, Zografi G. The importance of individual protein molecule dynamics in developing and assessing solid state protein preparations. Journal of pharmaceutical sciences. 2014;103(9):2605–14.
Pikal MJ, Rigsbee D, Roy ML, Galreath D, Kovach KJ, Wang B, et al. Solid state chemistry of proteins: II. The correlation of storage stability of freeze-dried human growth hormone (hGH) with structure and dynamics in the glassy solid. J Pharm Sci. 2008;97(12):5106–21.
Wang B, Tchessalov S, Cicerone MT, Warne NW, Pikal MJ. Impact of sucrose level on storage stability of proteins in freeze-dried solids: II. Correlation of aggregation rate with protein structure and molecular mobility. J Pharm Sci. 2009;98(9):3145–66.
Chang LL, Pikal MJ. Mechanisms of protein stabilization in the solid state. J Pharm Sci. 2009;98(9):2886–908.
Hvidt A, Nielsen SO. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386.
Englander SW, Kallenbach N. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q Rev Biophys. 1984;16:521–655.
Mayo SL, Baldwin RL. Guanidinium chloride induction of partial unfolding in amide proton exchange in RNase A. Science. 1993;262(5135):873–6.
Qian H, Mayo SL, Morton A. Protein hydrogen exchange in denaturant: quantitative analysis by a two-process model. Biochemistry. 1994;33(27):8167–71.
Dong A, Hyslop RM, Pringle DL. Differences in conformational dynamics of ribonucleases A and S as observed by infrared spectroscopy and hydrogen-deuterium exchange. Arch Biochem Biophys. 1996;333(1):275–81.
Sinha S, Li Y, Williams TD, Topp E. Protein conformation in amorphous solids by FTIR and by hydrogen/deuterium exchange with mass spectrometry. Biophys J. 2008;95(12):5951–61.
Mizuno M, Pikal MJ. Is the pre-Tg DSC endotherm observed with solid state proteins associated with the protein internal dynamics? Investigation of bovine serum albumin by solid state hydrogen/deuterium exchange. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2013;85(2):170–6.
Fang R, Grobelny PJ, Bogner RH, Pikal MJ. Protein internal dynamics associated with pre-system glass transition temperature endothermic events: investigation of insulin and human growth hormone by solid state hydrogen/deuterium exchange. J Pharm Sci. 2016;105(11):3290–5.
Parak F, Frolov EN, Kononenko AA, Mossbauer RL, Goldanskii VI, Rubin AB. Evidence for a correlation between the photoinduced electron transfer and dynamic properties of the chromatophore membranes from Rhodospirillum rubrum. FEBS Lett. 1980;117(1):368–72.
Ferrand M, Dianoux AJ, Petry W, Zaccai G. Thermal motions and function of bacteriorhodopsin in purple membranes: effects of temperature and hydration studied by neutron scattering. Proc Natl Acad Sci U S A. 1993;90(20):9668–72.
Rasmussen BF, Stock AM, Ringe D, Petsko GA. Crystalline ribonuclease A loses function below the dynamical transition at 220 K. Nature. 1992;357(6377):423–4.
Hill JJ, Shalaev EY, Zografi G. Thermodynamic and dynamic factors involved in the stability of native protein structure in amorphous solids in relation to levels of hydration. J Pharm Sci. 2005;94(8):1636–67.
Perez J, Zanotti JM, Durand D. Evolution of the internal dynamics of two globular proteins from dry powder to solution. Biophys J. 1999;77(1):454–69.
Cornicchi E, Marconi M, Onori G, Paciaroni A. Controlling the protein dynamical transition with sugar-based bioprotectant matrices: a neutron scattering study. Biophys J. 2006;91(1):289–97.
Zanotti JM, Bellissent-Funel MC, Parello J. Hydration-coupled dynamics in proteins studied by neutron scattering and NMR: the case of the typical EF-hand calcium-binding parvalbumin. Biophys J. 1999;76(5):2390–411.
Tsai AM, Neumann DA, Bell LN. Molecular dynamics of solid-state lysozyme as affected by glycerol and water: a neutron scattering study. Biophys J. 2000;79:2728–32.
Librizzi F, Viappiani C, Abbruzzetti S, Cordone L. Residual water modulates the dynamics of the protein and of the external matrix in “trehalose coated ” MbCO: an infrared and flash-photolysis study. J Chem Phys. 2002;116(3):1193–200.
Doster W, Cusack S, Petry W. Dynamic instability of liquidlike motions in a globular protein observed by inelastic neutron scattering. Phys Rev Lett. 1990;65(8):1080–3.
Hagen SJ, Hofrichter J, Eaton WA. Protein reaction kinetics in a room-temperature glass. Science. 1995;269:959–62.
Liao YH, Brown MB, Nazir T, Quader A, Martin GP. Effects of sucrose and trehalose on the preservation of the native structure of spray-dried lysozyme. Pharm Res. 2002;19(12):1847–53.
Chang L, Shepherd D, Sun J, Ouellette D, Grant KL, Tang XC, et al. Mechanism of protein stabilization by sugars during freeze-drying and storage: native structure preservation, specific interaction, and/or immobilization in a glassy matrix? J Pharm Sci. 2005;94(7):1427–44.
Bos OJ, Remijn JP, Fischer MJ, Wilting J, Janssen LH. Location and characterization of the warfarin binding site of human serum albumin: a comparative study of two large fragments. Biochem Pharmacol. 1988;37(20):3905–9.
Dong A, Prestrelski SJ, Allison SD, Carpenter JF. Infrared spectroscopic studies of lyophilization- and temperature-induced protein aggregation. J Pharm Sci. 1995;84(4):415–24.
Li Y, Williams TD, Schowen RL, Topp EM. Characterizing protein structure in amorphous solids using hydrogen/deuterium exchange with mass spectrometry. Anal Biochem. 2007;366(1):18–28.
Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J Res Bur Stand. 1977;81A:89–96.
Moorthy BS, Zarraga IE, Kumar L, Walters BT, Goldbach P, Topp EM, et al. Solid-state hydrogen–deuterium exchange mass spectrometry: correlation of deuterium uptake and long-term stability of lyophilized monoclonal antibody formulations. Mol Pharm 2017.
Forney-Stevens KM, Bogner RH, Pikal MJ. Addition of amino acids to further stabilize lyophilized sucrose-based protein formulations: I. screening of 15 amino acids in two model proteins. J Pharm Sci. 2016;105(2):697–704.
Fang R, Bogner RH, Nail SL, Pikal MJ. Stability of freeze-dried protein formulations: contributions of ice nucleation temperature and residence time in the freeze-concentrate. J Pharm Sci. 2020;109:1896–904.
Fang R, Tanaka K, Mudhivarthi V, Bogner RH, Pikal MJ. Effect of controlled ice nucleation on stability of lactate dehydrogenase during freeze-drying. J Pharm Sci. 2018;107(3):824–30.
Moorthy BS, Schultz SG, Kim SG, Topp EM. Predicting protein aggregation during storage in lyophilized solids using solid state amide hydrogen/deuterium exchange with mass spectrometric analysis (ssHDX-MS). Mol Pharm. 2014;11(6):1869–79.
Pikal MJ, Rigsbee DR, Roy ML. Solid state chemistry of proteins: I. glass transition behavior in freeze dried disaccharide formulations of human growth hormone (hGH). J Pharm Sci. 2007;96(10):2765–76.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to add the following affiliation as a second affiliation to Dr. Wasfy Obeidat. The second affiliation is Department of Pharmaceutical Technology, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan.
In Loving Memory of Professor Michael J. Pikal
Rights and permissions
About this article
Cite this article
Fang, R., Obeidat, W., Pikal, M.J. et al. Evaluation of Predictors of Protein Relative Stability Obtained by Solid-State Hydrogen/Deuterium Exchange Monitored by FTIR. Pharm Res 37, 168 (2020). https://doi.org/10.1007/s11095-020-02897-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02897-7