Abstract
Our growing understanding of membrane transporters and their substrate specificity has opened a new avenue in the field of targeted drug delivery. The L-type amino acid transporter 1 (LAT1) has been one of the most extensively investigated transporters for delivering drugs across biological barriers. The transporter is predominantly expressed in cerebral cortex, blood-brain barrier, blood-retina barrier, testis, placenta, bone marrow and several types of cancer. Its physiological function is to mediate Na+ and pH independent exchange of essential amino acids: leucine, phenylalanine, etc. Several drugs and prodrugs designed as LAT1 substrates have been developed to improve targeted delivery into the brain and cancer cells. Thus, the anti-parkinsonian drug, L-Dopa, the anti-cancer drug, melphalan and the anti-epileptic drug gabapentin, all used in clinical practice, utilize LAT1 to reach their target site. These examples provide supporting evidence for the utility of the LAT1-mediated targeted delivery of the (pro)drug. This review comprehensively summarizes recent advances in LAT1-mediated targeted drug delivery. In addition, the use of LAT1 is critically evaluated and limitations of the approach are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main aim of targeted drug delivery is to achieve therapeutic concentrations of the drug at the site of action in the tissue of interest to produce the desired pharmacological effect as well as minimizing the side effects caused by drug distribution to other organs. Delivery of the drug to the site where the target is located requires knowledge about the tissue and an understanding of what features the drug should possess in order to be selectively distributed. In addition to direct administration of the drug by some specific route to the target organ, several other strategies have been investigated including nanoparticle-based drug delivery systems, prodrugs or derivatives and stimuli sensitive targeted therapy (1,2).
One of the most promising approaches is the utilization of the transporters selectively expressed at the cell membrane of the target tissue (3,4). The design of drugs or prodrugs as substrates of the particular membrane transporter can enable targeted delivery of the drug based on the tissue specific expression profile of the carrier. In this respect, the L-type amino acid transporter 1 (LAT1, SLC7A5), which is expressed at a relatively high level at the blood-brain barrier (BBB), blood-retinal barrier (BRB), testis, bone marrow, placenta and several types of human tumour is an intriguing way for targeting a drug to these organs. Importantly, the transporter has already demonstrated its utility by mediating the brain delivery of the antiparkinsonian drug L-Dopa and the anticancer drug, melphalan (5,6). This suggests that effective drug delivery to the target organ expressing LAT1 can be achieved by rational design of the drugs mimicking the structures of the transporter endogenous substrates.
In the present review, the current state of the art and recent improvements in the LAT1-mediated drug delivery are summarized. In addition, the limitations and advantages of the approach are discussed.
LAT1 and its function
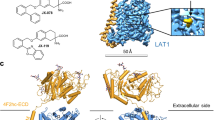
The L-type amino acid transporter 1 is a Na+- and pH-independent exchanger of large branched-chain and aromatic neutral amino acids with 1:1 stoichiometry (7,8). The transporter consists of two subunits covalently linked via a disulfide bond (9,10,11). The light chain subunit (LAT1, SLC7A5) is a functional subunit responsible for the exchange of amino acids (Fig. 1). The heavy chain subunit (known as CD98 or 4F2hc, SLC3A2) is a glycoprotein coupled with the light chain subunit which acts as a molecular chaperone localizing LAT1 at the plasma membrane. In addition, it is involved in the processes of cell survival and integrin activation. Recently, a cryo-electron microscopy study investigating the structure of the complex LAT1-4F2hc (hereinafter referred to as LAT1) revealed that in addition to a disulfide bond association, the light chain subunit cooperates extensively with 4F2hc on the extra- and intracellular side of the membrane as well as within the membrane (12). The authors concluded that the heavy chain subunit was crucial for the transport function of the complex (12).
The transporter is responsible for the inward flux of several essential amino acids such as phenylalanine, leucine, isoleucine, tryptophan, histidine, tyrosine in antiport with histidine, tyrosine and a non-essential amino acid glutamine (Fig. 1) (8,13,14,15). While the affinity of the essential amino acids for LAT1 is high (Km values for the human isoform range from 14 to 28 μM) (9,13,16), the non-essential glutamine showed lower affinity (Km 1.6 mM) to human LAT1. The intracellular accumulation of glutamine acting as an exchanger for essential amino acids is mediated by the transporters of system N and A (13,16,17). In addition to amino acid transport, LAT1 plays a role in the passage of thyroid hormones, i.e. triiodothyronine and thyroxine (18).
The LAT1 plays a crucial role in normal body functioning by regulating the exchange of the amino acids which take part in the synthesis of peptides, proteins and neurotransmitters as well as in nutrient metabolism. Thus, while developing LAT1-mediated drug delivery systems for targeting non-tumorous tissue, it is important that the vital function of the transporter should not be disturbed. In contrast, as the transporter plays a crucial role in cancer cell growth and survival, a strategy to affect the function and expression of LAT1 in a tumour has been investigated and considered as a promising treatment approach (19). More information regarding the structure, function, transport mechanism and homology modelling of LAT1 can be found in recently published studies and reviews (14,20,21,22,23,24,25,26).
Tissue expression of LAT1
The localization and mRNA/protein expression of the light chain subunit LAT1 in specific human tissues according to the Human Protein Atlas (27) is summarized in Table I. The mRNA expression of the LAT1 differs in the 27 studied human tissues with the highest expression present in the cerebral cortex, retina, esophagus, testis, placenta and bone marrow (27). LAT1 is primarily localized in the basolateral membrane of polarized epithelia (28,29). The highest levels of LAT1 protein expression of LAT1 were detected in parathyroid and adrenal glands, salivary gland, esophagus, bronchus, kidney, urinary bladder, bone marrow, appendix, testis, heart muscle, fallopian tube, cervix, gastrointestinal tract, smooth muscle and gallbladder (Table I).
In the endothelial cells of the brain microvessels, LAT1 is expressed on both apical and basolateral sides of the cell membranes (30). Additionally, LAT1 expression was detected in brain parenchymal cells such as human and mouse astrocytes, mouse and rat neurons and immortalized microglia cultures (31,32,33). LAT1 is also expressed in the inner BRB, ensuring the flux of large neutral amino acids and neurotransmitters (34).
Furthermore, LAT1 is overexpressed in human tumors such as cholangiocarcinoma, malignant glioma, multiple myeloma, and lung, bladder, bone, pancreas, thyroid, prostate, uterine cervical, breast cancer and other malignancies as compared to benign tissue used as the control (9,16,35,36,37). The association between LAT1 overexpression and meaningfully shorter survival in many types of cancer have indicated that the transporter may be exploited as a prognostic biomarker to predict the outcome in different cancer types (38,39).
The protein expression of LAT1 in the majority of the listed tissues has been also confirmed, although quantitative information about the absolute protein expression is still limited. Importantly, the protein expression of LAT1 can vary between species, which should be taken into account during the development of LAT1-utilizing (pro)drugs. For instance, in isolated cortex microvessels, the protein expression in rats (3.00 ± 0.62 fmol/μg protein) and mice (2.19 ± 0.21 fmol/μg protein) was higher than in humans (0.43 ± 0.09 fmol/μg protein) (40,41).
There is a correlation between the mRNA expressions of the heavy chain SLC3A2 and light chain SLC7A5 in human tissues. Similarly to SLC7A5, the highest levels of SLC3A2 mRNA expression were found in cerebral cortex, placenta, testis and bone marrow (27). This correlation hints at the presence of an interaction between the subunits. Additionally, high SLC3A2 expression has been detected in other tissues, i.e. parathyroid gland and kidney, where the heavy chain is responsible for localizing other SLC7 transporters at the cell membrane (27,29).
LAT1 and diseases
The expression and function of LAT1 can be altered in pathological conditions, such as cancer and central nervous system (CNS) diseases and consequently, this can lead to altered efficacy of LAT1-mediated drug delivery to the target organ.
Thus, overexpression of LAT1 in human brain metastasis in comparison to non-tumoral brains correlated to increased uptake of LAT1 substrate 6-[18F]-fluoro-L-3,4-dihydroxy-phenylalanine ([18F]-DOPA) (33). In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treated mouse model of Parkinson’s disease, mRNA expression of LAT1 at the BBB was significantly reduced, although this was not reflected in the protein expression level of the transporter (42). In addition, the loss of LAT1 due to mutations leads to impairment of amino acid homeostasis in the brain and therefore it has been suggested to be a cause of motor dysfunction and Autism Spectrum Disorder in mice and human (43). Recently, Gynther et al. (2018) showed that LAT1 function was not altered at the BBB of lipopolysaccharide (LPS) induced neuroinflammation murine model and in the transgenic Alzheimer’s disease model with amyloid precursor protein (APP) and presenilin (PSEN1) gene mutations, (31). In the same study, LAT1 protein expression and function were similar in wild type astrocytes with and without LPS treatment and in APP/PS1 transgenic astrocytes treated with LPS (31).
Furthermore, as previously mentioned due to upregulated LAT1 expression in several human cancers and its role in tumour cell growth and survival, the transporter has been considered as the potential target for anticancer therapy. The LAT1 inhibition as a strategy for cancer treatment has been summarized in several excellent reviews (14,19,20,21,44,45,46,47). In addition, Cibrian et al. (2020) demonstrated that LAT1 expression was upregulated in keratinocytes and skin infiltrating lymphocytes of psoriatic lesions in human subjects and mice (48). The authors considered targeting LAT1 as a potential immunosuppressive strategy to regulate skin inflammation driven by the interleukin IL-23/IL-1b/IL-17 axis (48).
Drug delivery via LAT1
Substrate specificity
One obstacle to the development of LAT1-utilizing compounds can be the potential inhibition of their uptake due to competition with amino acids. The essential amino acids are delivered into the brain mainly from the blood after dietary intake, while non-essential amino acids are synthetized inside the brain. Thus, the food or supplements containing high amounts of essential amino acids or protein can alter the delivery of LAT1 utilizing (pro)drugs. For instance, the brain uptake of L-Dopa, a substrate of LAT1, was decreased after a high-protein meal or the intravenous infusion of large neutral amino acids before the administration of the drug in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine parkinsonian monkeys (49). Nonetheless, the pilot study of Cucca et al. (2015) demonstrated that a six-month amino acid supplementation in protein-restricted patients with Parkinson’s disease, chronically treated with L-Dopa, did not affect neurological parameters (50).
The efficient utilization of LAT1 for drug delivery requires the rational design and development of compounds which can consequently compete with millimolar concentrations of amino acids. One of the strategies to overcome this issue is to develop (pro) drugs with higher affinity (Km) to LAT1 as compared to that of the endogenous amino acid substrates. In this respect, it is important to gather knowledge about substrate specificity and an understanding of ligand - transporter interactions. Despite the relatively low maximal transport rate (Vmax) enabling the substrate compounds to cross the cellular membrane per unit time, the amino acid-resembling (pro) drugs have demonstrated a potential to utilize the transporter for CNS and cancer delivery.
Several studies investigating the development of LAT1 substrate structure-activity relationship have been conducted (23,51,52,53,54,55). The findings suggest that binding to LAT1 requires the presence of both the amino and carboxylic acid functional groups as well as large, neutral side groups. In addition, the presence of aromaticity in the compound molecule, for instance in a prodrug promoiety such as phenylalanine, plays an important role in the binding to LAT1 and its ability to utilize transporter for cellular uptake (53,56,57).
Importantly, it has been demonstrated that the affinity (Km) to LAT1 of L-enantiomers of phenylalanine and leucine was higher as compared to the corresponding D-enantiomers. In contrast, the transport rate (Vmax) was similar for L and D-enantiomers (16). In the study of Chien et al. (2018), the authors concluded that LAT1 is not stereoselective in terms of the transport rate of the amino acids, whereas based on the relative IC50-values of L- and D-enantiomers of amino acid, there is variation in their binding to the transporter (58).
In addition, LAT1 has overlapping substrate specificity with other amino acid transporters, for instance LAT2 (20), which can complicate the development of LAT1 selective compounds for the targeted delivery.
Brain delivery of CNS-acting drugs
The comparatively high expression of LAT1 transporter on both the luminal and abluminal sides of the brain capillary endothelial cells as well as on brain parenchymal cells offers a promising opportunity for LAT1-mediated brain delivery of CNS drugs. The transporter has demonstrated its efficacy in the delivery of clinically used CNS drugs and prodrugs such as L-Dopa (Fig. 2A), baclofen (Fig. 2B), alpha-methyldopa (Fig. 2C) gabapentin (Fig. 2D) (5,6,59,60).
Chemical structures, molecular weight (MW) and logP of amino acid CNS drugs and derivatives designed to utilize LAT1 (part 1): L-Dopa (a), baclofen (b), alpha-methyldopa (c) gabapentin (d), derivative of phosphonoformate (e), derivative of nipecotic acid (f), derivative of L-tyrosine (g), derivatives of ketoprofen (h-l, n), derivative of 6-mercaptopurine (m), derivatives of valproic acid (o-u), derivatives of dopamine (v-x), derivative of benzoic acid (y). The values of logP were calculated using Marvin Sketch version 15.8.31 (ChemAxon, Budapest, Hungary)0
Several attempts have been made to develop LAT1-utilizing derivatives and prodrugs sharing the structural features of the natural substrates. Some derivatives have demonstrated efficient LAT1-mediated cellular uptake and/or BBB permeation after in situ brain perfusion in rats and mice (61,62,63,64,65,66). In the majority of cases, a parent drug has been conjugated to the amino acid side chain via a biodegradable linker in such a way that carboxyl and amino groups are not substituted to allow effective LAT1 binding (Figs. 2, 3).
Chemical structures, molecular weight (MW) and logP of amino acid CNS drugs and derivatives designed to utilize LAT1 (part 2): derivatives of perforin inhibitor (a, b), derivatives of ketoprofen (c-f, j), derivatives of ferulic acid (g, i), derivative of acyclovir (k). The values of logP were calculated using Marvin Sketch version 15.8.31 (ChemAxon, Budapest, Hungary).
Thus, the L-tyrosine derivative of antiviral phosphonoformate (Fig. 2E) synthetized by Walker et al. (1994) inhibited the uptake L-[3H]-tyrosine in porcine brain microvessel endothelial cells (67) providing evidence of LAT1 binding. A tyrosine conjugate of another CNS agent, nipecotic acid (Fig. 2F), designed as a substrate of the amino acid transporter displayed a concentration-dependent anticonvulsant effect in a murine epilepsy model - Diluted Brown Agouti/2 mice (68). In another study, p-nitro- and p-chlorobenzyl ether prodrugs of L-tyrosine (Fig. 2G) inhibited the uptake of L-[3H]-tyrosine in rabbit corneal cells (69). Gynther et al. (2008) demonstrated LAT1-mediated delivery of an L-tyrosine prodrug of ketoprofen (Fig. 2H) across the BBB using the in situ rat brain perfusion technique, as the brain uptake of the prodrug was significantly decreased after co-perfusion with the competitive LAT1 inhibitor, 2-amino-2-norbornanecarboxylic acid (BCH) (62). The ester- or amide-based ketoprofen prodrugs (Fig. 2I-L) conjugated to either phenylalanine or leucine did not show any binding to LAT1, as the brain uptake of L-[14C]-leucine was not altered after co-perfusion with these prodrugs in in situ brain perfusion in rats (62). This provides additional evidence that the presence of α-carboxyl and α-amino groups as well as a nonpolar side chain in the structure of substrate of the transporter is important for binding to LAT1. Killian et al. (2007) covalently conjugated L-cysteine via a disulphide bond to 6-mercaptopurine (Fig. 2M) and showed that the prodrug significantly inhibited the uptake of L-[14C]-leucine across the BBB after in situ brain perfusion in rats, thereby demonstrating affinity to LAT1 (63).
The neuropharmacokinetic study of the LAT1-utilizing L-lysine derivative of ketoprofen (Fig. 2N) using a cerebral microdialysis technique in rats revealed for the first time that after crossing the BBB, the prodrug was predominantly distributed into the brain intracellular compartment and subsequently this was followed by the release of the parent drug (61). Thus, the LAT1-mediated uptake of the prodrug was supported by the inhibition of the brain uptake of L-[14C]-leucine after in situ perfusion in rats, causing a reduction in the brain uptake of prodrug after co-perfusion with a LAT1 substrate, L-phenylalanine (61). The ratio of unbound parent drug AUC in brain extracellular fluid to plasma was more than doubled after prodrug dosing as compared to ketoprofen itself given as an i.v. bolus injection in rats (61). In addition, the estimated brain intracellular delivery efficacy evaluated as the AUC ratio of unbound parent drug in the brain intracellular compartment to plasma was significantly higher after prodrug dosing (21.8) in comparison to parent drug administration (0.06) (61). In another study, Peura et al. (2011) synthetized several LAT1-utilizing prodrugs of valproic acid and showed that meta-substituted amide- and ester-based phenylalanine derivatives (Fig. 2O, P) had 10-fold higher affinity to LAT1 in comparison with the para-substituted conjugates as demonstrated by inhibition of L-[14C]-leucine brain uptake by prodrugs after in situ brain perfusion in rats (Fig. 2Q, R) (65). Importantly, the amide-based meta-substituted prodrug (Fig. 2P) displayed 2-fold greater permeation rate across the BBB as compared to the para-substituted prodrug of valproic acid (Fig. 2R) as evaluated with in situ brain perfusion in rats (65). Furthermore, the ability to bind to LAT1 was investigated for these prodrugs (Fig. 2O-R) as well as for amide- and ester-based prodrugs of valproic acid (Fig. 2S,T) conjugated to phenylalanine with an additional methylene group and tryptophan prodrug of valproic acid (Fig. 2U) (70). The study demonstrated that the prodrugs inhibited the uptake of the LAT1 substrate L-[14C]-leucine in human breast cancer MCF-7 cells i.e. evidence of binding to LAT1. In addition, in pharmacokinetic study in rats, after a single i.v. bolus injection, amide-based prodrugs of valproic acid (Fig. 2P,R,S) crossed the BBB and released the parent drug, although the extent of brain delivery of valproic acid was not improved (70). Thus, the brain AUC and Cmax of the parent drug released after dosing with the prodrugs was more than 7 times lower than those after valproic acid administration (70). In another study, Peura et al. (2013) showed that the meta-substituted phenylalanine prodrug of dopamine (Fig. 2W) was able to cross the BBB using LAT1, as the prodrug significantly inhibited the brain uptake of L-[14C]-leucine and its own brain uptake was reduced after co-perfusion with L-phenylalanine after in situ brain perfusion in rat (64). In contrast, the aspartic and adipic acid conjugates of dopamine (Fig. 2V,X) did not reach the brain as assessed with in situ brain perfusion in rat (64). However, in comparison to L-Dopa dosing in rats, the developed phenylalanine prodrug (Fig. 2W) did not increase the levels of dopamine in the striatum after i.p. injection (64). The L-tryptophan prodrug of benzoic acid (Fig. 2Y) has been designed based on the three-dimensional (3D) quantitative structure–activity relationship (QSAR) analysis of LAT1 binding of substrates by using classical and topomer comparative molecular field analysis and the data from the in situ rat brain perfusion technique (53). The prodrug showed the ability to inhibit the brain uptake of L-[14C]-leucine using in situ brain perfusion in rat (53).
The developed perforin inhibitor ester- (Fig. 3A) and amide-based (Fig. 3B) derivatives inhibited L-[14C]-leucine brain uptake after the in situ mouse brain perfusion (71). However, their brain uptake was not inhibited after co-perfusion with LAT1 substrate, L-tryptophan, suggesting utilization of transporter(s) other than LAT1. Interestingly, the cellular uptake of both derivatives was doubled by L-tryptophan in MCF-7 cells, providing additional evidence that the transporters other than LAT1 can be involved in their uptake (72). The cell uptake kinetics analysis, performed in MCF-7 cells, revealed involvement of two transporters, i.e. high-affinity low-capacity transporter and low-affinity high-capacity transporter (72). Interestingly, the uptake of ester-based prodrug of perforin inhibitor (Fig. 3A) into primary mouse neurons and immortalized microglia was reduced by approximately 25% by LAT1 inhibitor (KMH-233), while the uptake of this compound to mouse astrocytes decreased by around 70% by LAT1 inhibitor (74). Both prodrugs reached the brain after a single dose administered by i.p. injection in mice, while administration of the parent drugs did not result in BBB permeation. In addition, the parent drug was detected in the brain only after dosing with the ester-based prodrug (Fig. 3A) (71). Interestingly, the released parent drug was not detected in liver after a single dose i.p. injection of both prodrugs in mice. The ratio of liver AUC to plasma AUC for prodrugs (Fig. 3A, B) was significantly lower than that for parent drugs after a single dose i.p. injection in mice (71).
Recently, the structure-pharmacokinetics relationship of five derivatives of ketoprofen (Fig. 2N, Fig. 3C, D, E, F) designed to utilize LAT1 was investigated (57). The study confirmed the previous findings that the aromaticity presented in the prodrug’s promoiety plays a significant role in affinity to LAT1 and its utilization for cellular uptake of prodrugs. Thus, phenylalanine derivatives (Fig. 3C, D, E) but not derivatives with aliphatic promoieties, (Fig. 2N, Fig. 3F) significantly inhibited the uptake of L-[14C]-leucine in human retinal pigmented epithelial cells ARPE-19 cells. However, the uptake of all five derivatives was significantly inhibited by the LAT1 inhibitor, KMH-233 (66). Importantly, while all derivatives showed an ability to cross the mouse BBB according to in situ brain perfusion and could detected after a single i.p. injection of compounds in mice, the released parent drug was quantified in the brain only after administration of phenylalanine derivatives (Fig. 3C, D). The ratios of AUCs for the brain to plasma for unbound released ketoprofen from meta- and para-substituted phenylalanine derivatives (0.13 and 0.35, respectively) were significantly higher as compared to ketoprofen dosing (0.01) (66). In addition, five times higher ratios of AUCs for the brain to liver of ketoprofen released from these prodrugs were estimated as compared to ketoprofen i.p. dosing in mice (66). Thus, the study revealed that meta- or para-conjugation of phenylalanine directly to the parent drug molecule such as ketoprofen is an important structural feature which can provide targeted brain delivery of ketoprofen with reduced systemic exposure to the released parent drug. Importantly, an intra-brain distribution study of meta-conjugated phenylalanine prodrug of ketoprofen (Fig. 3C) using the brain slice method in mice and rats revealed a predominantly LAT1-mediated delivery of this prodrug to the intracellular compartment of the brain parenchyma in mice and rats (73). Thus, these findings suggest that in addition to reduced peripheral exposure to released parent drug, this prodrug enhanced the delivery of ketoprofen to the intracellular compartment of the brain parenchyma where the target, cyclooxygenase, is located. In addition, it has been demonstrated for the first time that the intra-brain distribution of LAT1-utilizing prodrug of ketoprofen (Fig. 3C) did not affect the transporter function such as amino acid transport and protein expression as shown in brain slices of mice and rats (73). In addition, Huttunen et al. (2019) reported that this prodrug accumulated in primary neurons and astrocytes, as well as in immortalized microglia in vitro (74). However, the role of LAT1 in the uptake of the prodrug to these cells was not confirmed. Moreover, the concentrations of the prodrug used in the study were higher than its Km value determined in the uptake studies in ARPE-19 cells (57) suggesting that at these concentrations the prodrug can utilize other transporter(s). Importantly, the findings of this structure-pharmacokinetic relationship analysis (57) and 3D QSAR (53) were confirmed in the study of derivatives developed to improve brain delivery of natural phenolic compound ferulic acid. The results revealed that the amide-based meta-conjugated phenylalanine derivatives of this agent (Fig. 2G, H) were efficiently bound to LAT1 and utilized the transporter for cellular uptake as well as permeating into the mouse brain after i.p. injection (56). However, the delivery of ferulic acid has not been improved after the dosing of these derivatives, as the AUC and Cmax of the released ferulic acid in the brain after a single dose i.p. injection of meta-substituted phenylalanine derivative was more than three times lower than after parent drug administration (56).
In the studies of Puris et al. (2019) (56) and Huttunen et al. (2018) (75), the change of the linker from amide (Fig. 3C,G) to ester bond between the promoiety (Fig. 3I, J) and parent drugs (ferulic acid and ketoprofen, respectively), resulted in a loss of LAT1 substrate specificity in vitro. Therefore, the design of prodrugs/derivatives with either ester-or amide-linker needs to be justified for each parent drug on a case by case basis.
All in all, the results of these studies illustrate that LAT1 natural substrates might serve as a promising template for improving the delivery of drugs into the brain. However, the review revealed that only a few studies have demonstrated LAT1-mediated BBB permeation (61,62,64), i.e. for most of the compounds, it is only the affinity to LAT1 which has been investigated, either using in situ brain perfusion or in vitro cellular uptake inhibition experiments with LAT1 substrates. In addition, information about the intra-brain distribution has been provided only for two compounds (Fig. 2H, Fig. 3C). Moreover, there is a lack of the knowledge about the distribution to other tissues and therefore it is difficult to evaluate the brain targeting via LAT1, as only two studies (57,71) measured the distribution of (pro) drugs to liver and compared it to the brain distribution. Therefore, future studies should focus on more complex investigations of the delivery via LAT1 across the BBB, the cellular barrier of the brain parenchyma and other organs.
Ocular delivery via LAT1
In conjunction with the improvement of the transport across the BBB, LAT1-mediated delivery has been used to circumvent poor permeability across the BRB. The LAT1 is expressed in the retina, where it is responsible for the exchange of amino acids (76,77) offering the opportunity for LAT1-mediated delivery of the drugs. Thus, several amino acid ester prodrugs of acyclovir have been developed, and the permeation of L-serine derivative of acyclovir (Fig. 3K) across cornea was inhibited by arginine and BCH, pointing to the involvement of cationic amino acid transporter 1 and LAT1 in its transport (78,79). Moreover, the derivative of acyclovir led to higher concentrations of acyclovir being released in the aqueous humour as compared to the parent drug in a study using a combination of the topical well infusion and aqueous humour microdialysis techniques in rabbits (79). Akanuma et al. (2018) demonstrated that [3H]-gabapentin utilized LAT1 for passage across the BRB in rats after carotid artery injection as well as cellular uptake in vitro in the models of the inner (TR-iBRB2 cells) and outer BRB (rat retinal pigment epithelial RPE-J cells), since the uptake of the compound was inhibited by LAT1 substrates (80). These studies hold promise that LAT1-mediated (pro) drug delivery could be used not only for the brain targeting, but also for the passage of the compounds across other LAT1 expressing barrier tissues such as BRB. However, additional studies will be required to investigate the efficiency of this strategy for the BRB delivery.
Delivery of anticancer agents
Due to the significant up-regulation of LAT1 expression in several human tumors, LAT1 has been an attractive transporter for targeted delivery of amino acid-derived anticancer drugs and prodrugs as well as positron emission tomography (PET) probes for cancer diagnosis. In this chapter, we will summarize the studies focusing on the development of LAT1-utilizing compounds targeting cancer cells.
Delivery of anticancer (pro)drugs
LAT1 has been shown to be involved in transport of anticancer drugs such as melphalan (Fig. 4A) and acivicin (Fig. 4B). The L-phenylalanine prodrug melphalan (Fig. 4A), a widely used anticancer drug for the treatment of ovarian cancer and multiple myeloma, inhibited the uptake of L-leucine into human MDA-MB-231 breast cancer cells (81), T24 human bladder carcinoma cells (82) and in hLAT1 expressing Xenopus oocytes (16) providing evidence of binding to LAT1. Melphalan at therapeutic doses reduced the total number of cells and the live-to-dead cell ratio in two esophageal adenocarcinoma cell lines, i.e. Bic-1 and Seg-1, and in the SV-40-immortalized esophageal squamous cell line, Het-1A (83). The sensitivity to the effects of melphalan decreased after simultaneous incubation with the competitive LAT1 inhibitor BCH, demonstrating LAT1-mediated cellular uptake of the drug in the above-mentioned cell lines. Moreover, the transporter mediated passage of melphalan across the rat BBB after in situ brain perfusion (84). As melphalan is used for anti-cancer chemotherapy in patients with retinoblastoma, Hosoya et al. (2008) investigated the LAT1 binding of melphalan and other synthetic amino acid-conjugated mustards in LAT1 expressing conditionally immortalized rat retinal endothelial TR-iBRB2 cells (85). The study showed that melphalan inhibited the uptake of [3H]-phenylalanine, evidence in support of its binding to LAT1 (85). Furthermore, Hosoya et al. (2008) demonstrated that while lysine-mustard (Fig. 4C) and aromatic amino acid-mustards (Fig. 4D, E) significantly inhibited [3H]-phenylalanine uptake in TR-iBRB2 cells, aliphatic amino acid-mustards, such as alanine-mustard (Fig. 4F) and ornithine-mustard (Fig. 4G), did not bind to LAT1 (85). Interestingly, the analogue of melphalan, a nitrogen mustard amino acid D,L-2-amino-7-bis[(2-chloroethyl)amino]-l,2,3,4-tetrahydro-2-naphthoic acid (D,L-NAM) (Fig. 4H), utilized LAT1 for cellular uptake into murine L1210 lymphocytic leukaemia cells (86) and exhibited 50-times higher affinity for LAT1 at the rat BBB than melphalan after in situ brain perfusion (87). As a result, the BBB penetration of D,L-NAM in rats was more than 20 times greater than that of melphalan after in situ brain perfusion (87). In another study, several isomers (C-6 and C-8) of D,L-NAM (C-7 isomer) were synthetized in order to improve the affinity of the agent (88). However, when investigated using in situ rat brain perfusion, it was found that all compounds had lower affinity to LAT1 as compared to D,L-NAM.
Chemical structures, molecular weight (MW) and logP of amino acid anticancer drugs and derivatives designed to utilize LAT1: melphalan (a), acivicin (b), derivatives of mustards (c-g), D,L-2-amino-7-bis[(2-chloroethyl)amino]-l,2,3,4-tetrahydro-2-naphthoic acid (h), derivative of gemcitabine (i), derivative of doxorubicin (j) derivative of methotrexate (k), 3CDIT (l). The values of logP were calculated using Marvin Sketch version 15.8.31 (ChemAxon, Budapest, Hungary).
LAT1 is involved in transport of another anticancer drug, acivicin (Fig. 4B), as demonstrated in a trans-stimulation assay of L-leucine efflux in human embryonic kidney HEK-LAT1 cells (23). However, the drug has failed due to unacceptable CNS toxicity caused by the high distribution of the compound to the brain. In cats, the toxic CNS effects could be reduced after concomitant administration of acivicin and a mixture of four large neutral amino acids or Aminosyn (mixture of 16 amino acids), i.e. evidence for the involvement of LAT1 in the brain distribution of the drug (89).
Another attempt to apply an LAT1-mediated delivery approach for cancer targeting has been conducted by Hong et al. (2018) who developed a threonine-derivative of gemcitabine (Fig. 5I) (90). The compound evoked a greater cytotoxic effect in LAT1-overexpressing human pancreatic cancer cells, BxPC-3 and MIAPaCa-2, as compared to the parent drug (89). However, no evidence of LAT1-utilization by the derivative was reported; this compound does not fulfil the structural requirements of a LAT1 substrate. In another study, an aspartate derivative of doxorubicin (Fig. 4J) inhibited the uptake of L-[3H]-leucine in the S2-LAT1 transgene cell line demonstrating binding to the transporter (91). Importantly, in human liver cancer HepG2 (LAT1-positive) tumor-bearing mice, this derivative exerted a significantly stronger inhibition of tumour growth as compared to doxorubicin treatment (91). Singh et al. (2016) applied the LAT1-mediated prodrug approach to improve delivery of methotrexate to a brain tumour by conjugating the drug to lysine (Fig. 4K) via a carboxylic acid group (92). The compound demonstrated a four times greater distribution of released methotrexate between brain and plasma in comparison to that encountered after the i.v. administration of the parent drug in mice (92). However, the involvement of LAT1 in delivery of the conjugate was not investigated. Moreover, a biodistribution study in mice with i.v. administration of both the stable radiolabelled parent drug and conjugate showed that both compounds were widely distributed to many tissues including liver, spleen, lung and kidney (92).
Chemical structures, molecular weight (MW) and logP of amino acid-based PET tracers L-[11C]-methyl-methionine (A), O-(2-[18F]-fluoroethyl)-L-tyrosine (B), 6-[18F]-fluoro-L-3,4-dihydroxy-phenylalanine (C), L-3-[18F]-fluoro-α-methyl tyrosine (D), (S)-2-amino-3-[3-(2-[18F]-fluoroethoxy)-4-iodophenyl]-2-methylpropanoic acid (E) as well as p-borono-phenylalanine (F). The values of logP were calculated using Marvin Sketch version 15.8.31 (ChemAxon, Budapest, Hungary).
There are several studies describing the potential of LAT1 utilization for enhanced delivery of nanoparticles and liposomes into cancer cells. Thus, An et al. (2016) intercalated doxorubicin into the adenosine-5′-triphosphate (ATP) responsive DNA scaffold, which was subsequently condensed and protected with a glutathione responsive polymer pOEI and functionalized with the LAT1 substrate 3CDIT (Fig. 4L) responsible for targeting the drug to tumour cells (93). The 3CDIT-targeting pOEI/doxorubicin/ATP aptamer nanoparticles demonstrated an outstanding accumulation in human-derived malignant glioma cells U87. Moreover, there was enhanced accumulation and doxorubicin release at the glioma tumour site as well as an improved antitumor therapeutic effect after i.v. injection of fluorescent-labelled nanoparticles in glioma model nude mice without any signs of systemic toxicity (93). In another study, Bhunia et al. (2017) developed LAT1 a selective liposomal drug carrier prepared from a novel L-Dopa functionalized amphiphile (Amphi-Dopa) (94). In the biodistribution study, the NIR-dye labelled liposomes of Amphi-Dopa were detected in the brain, liver and spleen at 4 h after i.v. administration but predominantly in the brain at 24 h in glioblastoma-bearing mice (94). The i.v. administration of the liposomes encapsulated with WP-1066, a potent signal transducer and activator of transcription 3 inhibitor, increased the overall survivability of mice bearing orthotopically established mouse glioblastoma dramatically (by ∼60%) as compared to that for the untreated mouse group (94).
Conjugation of liposomes and nanoparticles to glutamate has been used for improving the delivery of paclitaxel (95) and docetaxel (96) to cancer cells. The uptake of the liposomes in LAT1-expressing glioma cells was inhibited by leucine and phenylalanine only by 20–25% (96). The uptake of the nanoparticles was higher in LAT1-overexpressing cells (human cervix epitheloid carcinoma HeLa cells and MCF-7 cells) when compared to the situation in a LAT1 non-expressing cell line from Mouse Swiss NIH embryo NIH 3 T3 cells (95). Although based on these findings, the authors suggested LAT1-mediated delivery of the developed liposomes and nanoparticles, there is insufficient evidence for the involvement of LAT1 in the delivery of glutamine conjugates. In another study, the conjugation of L- and D-Dopa conjugated anisotropic gold nanoparticles increased the accumulation in several breast cancer cell lines (MCF-7, MDA-MB-231, MDA-MB-468, and MDA-MB-453) as compared to non-targeted nanoparticles conjugated with dopamine and 4-ethylcatechol (97). Moreover, the authors demonstrated LAT1-mediated accumulation of the L-Dopa functionalized anisotropic gold nanoparticles in MCF-7 cells, and the uptake was significantly inhibited by phenylalanine (97).
Delivery of PET probes for cancer imaging
The knowledge of LAT1 overexpression in tumours and its substrate specificity has been exploited in the development of radiolabelled probes, transporter substrates, used in cancer diagnostics. The labelling of [18F] or [11C] in the LAT1 substrate structure allows PET imaging of accumulated compounds in cancer foci after their administration. Thus, several amino acids based probes have been developed including (S)-2-amino-3-[3-(2-[18F]-fluoroethoxy)-4-iodophenyl]-2-methylpropanoic acid ([18F]-FIMP), L-3-[18F]-fluoro-α-methyl tyrosine ([18F]-FAMT), 6-[18F]-fluoro-L-3,4-dihydroxy-phenylalanine ([18F]-DOPA), L-[11C]-methyl-methionine ([11C]-MET) and O-(2-[18F]-fluoroethyl)-L-tyrosine ([18F]-FET). [11C]-MET (Fig. 5A) has been the most commonly used radiolabelled amino acid due to its convenient and rapid synthesis (98) and high specificity in tumour detection, delineation and in the differentiation of benign from malignant lesions (99). In terms of LAT1-mediated delivery, Okubo et al. (2010) revealed that uptake of [11C]-MET in human newly diagnosed gliomas was correlated with the extent of LAT1 expression (100). Later, the development of another amino acid-based PET tracer [18F]-FET (Fig. 5B), a tyrosine analogue with a longer half-life of 18F, meant that it was possible to use this tracer in centres with no access to a cyclotron. The tracer provided information comparable to that obtained with [11C]-MET in the diagnostics of gliomas and brain metastases (101). Non-labelled FET induced approximately 1% efflux of preinjected phenylalanine in a trans-stimulation study in Xenopus laevis oocytes expressing h4F2hc-hLAT1 (102) indicative of low extracellular affinity to LAT1 or a slow transport rate of FET. In contrast, Habermeier et al. (2015) showed that extracellular FET stimulated the efflux of intracellular L-[3H]-leucine in Xenopus laevis oocytes expressing human h4F2hc-hLAT1, while preinjected FET to the oocytes did not stimulate L-[3H]-leucine influx into the cells, interpreted to mean that FET was an influx, but not an efflux LAT1 substrate (103). The discrepancy between the study findings of Lahoutte et al. (2004) and Habermeier et al. (2015) can be explained by the different transport kinetics of LAT1 substrates, phenylalanine and leucine, used in these studies (13). In addition, in the study of Habermeier et al. (2015), FET significantly inhibited the uptake of L-[3H]-tyrosine in LN229 glioblastoma cells suggesting that it was utilizing LAT1 as it accumulated in the oocytes expressing human h4F2hc-hLAT1, but not in control non-expressing LAT1 oocytes (103). The PET tracer [18F]-DOPA (Fig. 5C) has been widely used for research in the field of neuroendocrine tumours and movement disorders; it has been demonstrated an efficacy comparable to [11C]-MET for detecting brain tumors (104). In another study, the compound exhibited the best performance for the diagnostic of recurrent medullary thyroid carcinoma as compared to [11C]-MET, 2-deoxy-2-[18F]-fluoro-d-glucose ([18F]-FDG), Ga-somatostatin analogues and 3-O-methyl-6-[18F]-fluoro-DOPA (105). The uptake of [18F]-DOPA in biopsy samples of patients with newly diagnosed astrocytoma correlated with LAT1 expression (106). Tomiyoshi et al. (1997) synthetized an amino acid probe, [18F]-FAMT (Fig. 5D), which significantly inhibited LAT1-mediated uptake of L-[14C]-leucine and stimulated the efflux of preloaded L-[14C]-leucine indicating binding to LAT1 (107). In addition, the compound utilized the transporter for uptake in mouse renal proximal tubule cell line S2 stably expressing hLAT1 (108). Moreover, the uptake of [18F]-FAMT in the tumour correlated with the LAT1 expression level in patients with non–small-cell lung cancer (109) and oral squamous cell carcinoma (110). Recently, Nozaki et al. (2019) designed and synthetized a phenylalanine based tracer, [18F]-FIMP (Fig. 5E), which demonstrated greater tumor targeted delivery in the subcutaneous LAT1-positive human glioblastoma xenograft model as compared to [18F]-FET, [11C]-MET and [18F]-FDG (111).
Boron neutron capture therapy
Targeting of anticancer agents via LAT1 has been applied for boron neutron capture therapy (BNCT) with the focus on patients suffering from high grade glioma (112,113). BNCT is a radiotherapy which is based on a nuclear fission reaction that occurs when 10B is irradiated with low energy thermal neutron beams and high-energy alpha-particles (4He2+) and recoiling lithium (7Li) nuclei (114). The efficacy of the BNCT is dependent on 10B accumulation in cancer tissue, which can be improved via LAT1-mediated delivery. Thus, p-borono-phenylalanine (BPA, Fig. 5F), a boron compound widely used in BNCT, demonstrated higher affinity to LAT1 as compared to LAT2 and ATB0,+ in Xenopus laevis oocytes expressing human h4F2hc-hLAT1 (115). However, the authors assumed that at therapeutic doses, the compound would be able to utilize both LAT1 and ATB0,+ for its uptake into cancer tissue (115).
Overall, these studies indicate that it should be possible to develop LAT1 selective compounds to be delivered via this transporter to cancer tissue for use in chemotherapy and cancer diagnostics. In this respect, a more systematic investigation of LAT1-mediated delivery and also a deeper understanding distribution of the compounds to healthy tissues are vital for the evaluation of the usefulness of the approach.
Opportunities and limitations of LAT1-mediated drug delivery
The LAT1- mediated delivery is a promising approach, which has found applications not only in improving delivery of drugs, but also for diagnostic purposes. However, as the present review demonstrated, although a great number of (pro) drugs have been developed to improve targeted delivery via LAT1, only a limited number of LAT1-utilizing agents have entered clinical trials or are currently being used in clinical practice. In addition, only a few studies have demonstrated the utilization of LAT1 for delivery of investigated compounds; most of the studies lack evidence of LAT1-mediated uptake of the (pro) drugs or agents, which complicates the evaluation of the effectiveness of this approach. Furthermore, as there is no standardized procedure(s) for the evaluation of transporter-mediated delivery, the use of different methods (in vitro, in situ and in vivo) in the studies makes it difficult to compare the results such as transport kinetics parameters and distribution to target tissue.
There are several limitations of the approach which should be considered during the development of LAT1-utilizing (pro) drugs and agents. First, this delivery method is applicable for small-molecule drugs and not suitable for the delivery of macromolecules. The molecular weight of the summarized pro (drugs) with reported affinity to LAT1 and transporter-mediated uptake ranged between 148.21–513.56 g/mol. In the case of LAT1-mediated delivery of nanoparticles and liposomes, it seems evident that LAT1 plays a role in directing the nano-carrier to the cells expressing the transporter through the binding to LAT1 rather than delivering the nanoparticle via the transporter. Second, as discussed above (chapter “Brain delivery of CNS-acting drugs”), the LAT1-mediated (pro) drug approach provides predominant intracellular distribution of the substrates and therefore can benefit the delivery of the drugs with intracellular, but not extracellular targets. In addition, the affinity of (pro) drugs to LAT1 should be sufficient to be able to compete with natural substrates for LAT1 binding, but not enough to interfere with their homeostasis in the case of the non-cancerous tissue targeting. The review demonstrated that only limited information regarding the effect of LAT1-utilizing (pro) drugs on amino acid homeostasis is available; clearly future studies should address this issue.
Finally, one should remember that absolute targeting via LAT1 to a particular organ cannot be achieved due to the expression of LAT1 in several tissues and its overlapping substrate specificity with other transporters. The present review revealed that only a few studies have addressed the issue of targeting via LAT1 and investigated the systemic distribution of the compounds. Therefore, future research should focus on investigating the distribution of LAT1-utilizing (pro) drugs and agents to other tissues, in particular for cancer targeting. Thus, the selection of LAT1-mediated drug delivery should be carefully considered for each compound taking into account the advantages of the approach and its limitations.
Conclusions
LAT1-mediated drug delivery is a promising approach, which has demonstrated its effectiveness for the brain- and cancer-targeted delivery of several agents, and it might be potentially used for delivery to other LAT-expressing tissues. In addition, several promising compounds have been developed and demonstrated the ability to bind to LAT1 and reach the target tissue in animal models. However, this review revealed that there is a lack of systematic knowledge of the efficiency of LAT1-mediated delivery in vivo, the distribution of compounds to non-target tissues, the proof of pharmacodynamic efficacy in disease models and translation of the data to humans. Therefore, further studies will be required to shed light on these issues if there is to be effective utilization of this approach in the targeted delivery of therapeutic compounds.
Abbreviations
- APP :
-
Amyloid precursor protein
- ATB0,+ :
-
b(0,+)-Type amino acid transporter
- ATP:
-
Adenosine triphosphate
- AUC:
-
Area under the total plasma or tissue concentration–time curve
- BBB:
-
Blood-brain barrier
- BCH:
-
2-amino-2-norbornanecarboxylic acid
- BNCT:
-
Boron neutron capture therapy
- BPA:
-
p-borono-phenylalanine
- BRB:
-
Blood-retinal barrier
- Cmax :
-
Maximum total concentration of the drug achieved in the plasma or tissue
- CNS:
-
Central nervous system
- D,L-NAM:
-
D,L-2-amino-7-bis[(2-chloroethyl)amino]-l,2,3,4-tetrahydro-2-naphthoic acid
- DNA:
-
Deoxyribonucleic acid
- IC50 :
-
Concentration of an inhibitor at which the response (or binding) is reduced by half
- IL:
-
Interleukins
- i.p.:
-
Intraperitoneal route of administration
- i.v.:
-
Intravenous route of administration
- K m :
-
Constant of Michaelis-Menten kinetics
- LAT:
-
L-type amino acid transporter
- LPS:
-
Lipopolysaccharide
- mRNA:
-
Messenger ribonucleic acid
- PET:
-
Positron emission tomography
- PSEN1 :
-
Presenilin 1
- QSAR :
-
Quantitative structure-activity relationship
- SLC:
-
Solute carrier transporters
- SV-40:
-
Simian virus 40
- V max :
-
Maximal transport rate
- 3D:
-
Three-dimensional space
- [11C]-MET:
-
L-[11C]-methyl-methionine
- [18F]-DOPA:
-
6-[18F]-fluoro-L-3,4-dihydroxy-phenylalanine
- [18F]-FAMT:
-
L-3-[18F]-fluoro-α-methyl tyrosine
- [18F]-FET:
-
O-(2-[18F]-fluoroethyl)-L-tyrosine
- [18F]-FIMP:
-
(S)-2-amino-3-[3-(2-[18F]-fluoroethoxy)-4-iodophenyl]-2-methylpropanoic acid
References
Kim SM, Faix PH, Schnitzer JE. Overcoming key biological barriers to cancer drug delivery and efficacy. J Control Release. 2017;267:15–30.
Miller G. Drug targeting. Breaking down barriers. Science. 2002;297(5584):1116–8.
Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14.
Sutera FM, De Caro V, Giannola LI. Small endogenous molecules as moiety to improve targeting of CNS drugs. Expert Opin Drug Deliv. 2017;14(1):93–107.
Goldenberg GJ, Lam HY, Begleiter A. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J Biol Chem. 1979;254(4):1057–64.
Kageyama T, Nakamura M, Matsuo A, Yamasaki Y, Takakura Y, Hashida M, et al. The 4F2hc/LAT1 complex transports L-DOPA across the blood-brain barrier. Brain Res. 2000;879(1–2):115–21.
Oxender DL, Christensen HN. Evidence for two types of mediation of neutral and amino-acid transport in Ehrlich cells. Nature. 1963;197:765–7.
Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70(1):43–77.
Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998;273(37):23629–32.
Verrey F, Jack DL, Paulsen IT, Saier MH Jr, Pfeiffer R. New glycoprotein-associated amino acid transporters. J Membr Biol. 1999;172(3):181–92.
Verrey F, Meier C, Rossier G, Kuhn LC. Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Pflugers Arch. 2000;440(4):503–12.
Yan R, Zhao X, Lei J, Zhou Q. Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature. 2019;568(7750):127–30.
Meier C, Ristic Z, Klauser S, Verrey F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002;21(4):580–9.
Scalise M, Galluccio M, Console L, Pochini L, Indiveri C. The human SLC7A5 (LAT1): the intriguing Histidine/large neutral amino acid transporter and its relevance to human health. Front Chem. 2018;6:243.
Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, et al. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395(6699):288–91.
Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514(2):291–302.
O’Kane RL, Vina JR, Simpson I, Hawkins RA. Na+ −dependent neutral amino acid transporters a, ASC, and N of the blood-brain barrier: mechanisms for neutral amino acid removal. Am J Physiol Endocrinol Metab. 2004;287(4):E622–9.
Friesema EC, Docter R, Moerings EP, Verrey F, Krenning EP, Hennemann G, et al. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology. 2001;142(10):4339–48.
Jin SE, Jin HE, Hong SS. Targeting L-type amino acid transporter 1 for anticancer therapy: clinical impact from diagnostics to therapeutics. Expert Opin Ther Targets. 2015;19(10):1319–37.
Kandasamy P, Gyimesi G, Kanai Y, Hediger MA. Amino acid transporters revisited: new views in health and disease. Trends Biochem Sci. 2018;43(10):752–89.
Singh N, Ecker GF. Insights into the Structure, Function, and Ligand Discovery of the Large Neutral Amino Acid Transporter 1, LAT1. Int J Mol Sci. 2018;19(5).
Singh N, Villoutreix BO, Ecker GF. Rigorous sampling of docking poses unveils binding hypothesis for the halogenated ligands of L-type amino acid transporter 1 (LAT1). Sci Rep. 2019;9(1):15061.
Geier EG, Schlessinger A, Fan H, Gable JE, Irwin JJ, Sali A, et al. Structure-based ligand discovery for the large-neutral amino acid transporter 1, LAT-1. Proc Natl Acad Sci U S A. 2013;110(14):5480–5.
Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, et al. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol. 2002;61(4):729–37.
Lee Y, Wiriyasermkul P, Jin C, Quan L, Ohgaki R, Okuda S, et al. Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat Struct Mol Biol. 2019;26(6):510–7.
Napolitano L, Galluccio M, Scalise M, Parravicini C, Palazzolo L, Eberini I, et al. Novel insights into the transport mechanism of the human amino acid transporter LAT1 (SLC7A5). Probing critical residues for substrate translocation. Biochim Biophys Acta Gen Subj. 2017;1861(4):727–36.
Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13(2):397–406.
Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447(5):532–42.
Fotiadis D, Kanai Y, Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol Asp Med. 2013;34(2–3):139–58.
Duelli R, Enerson BE, Gerhart DZ, Drewes LR. Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab. 2000;20(11):1557–62.
Gynther M, Puris E, Peltokangas S, Auriola S, Kanninen KM, Koistinaho J, et al. Alzheimer's disease phenotype or inflammatory insult does not Alter function of L-type amino acid transporter 1 in mouse blood-brain barrier and primary astrocytes. Pharm Res. 2018;36(1):17.
Papin-Michault C, Bonnetaud C, Dufour M, Almairac F, Coutts M, Patouraux S, et al. Study of LAT1 expression in brain metastases: towards a better understanding of the results of positron emission tomography using amino acid tracers. PLoS One. 2016;11(6):e0157139.
Wittmann G, Mohacsik P, Balkhi MY, Gereben B, Lechan RM. Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice. Fluids Barriers CNS. 2015;12:21.
Tomi M, Mori M, Tachikawa M, Katayama K, Terasaki T, Hosoya K. L-type amino acid transporter 1-mediated L-leucine transport at the inner blood-retinal barrier. Invest Ophthalmol Vis Sci. 2005;46(7):2522–30.
Imai H, Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30(12):4819–28.
Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, et al. Expression of L-type amino acid transporter 1 (LAT1) in neuroendocrine tumors of the lung. Pathol Res Pract. 2008;204(8):553–61.
Wang Q, Tiffen J, Bailey CG, Lehman ML, Ritchie W, Fazli L, et al. Targeting amino acid transport in metastatic castration-resistant prostate cancer: effects on cell cycle, cell growth, and tumor development. J Natl Cancer Inst. 2013;105(19):1463–73.
Maimaiti M, Sakamoto S, Yamada Y, Sugiura M, Rii J, Takeuchi N, et al. Expression of L-type amino acid transporter 1 as a molecular target for prognostic and therapeutic indicators in bladder carcinoma. Sci Rep. 2020;10(1):1292.
Yanagisawa N, Ichinoe M, Mikami T, Nakada N, Hana K, Koizumi W, et al. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J Clin Pathol. 2012;65(11):1019–23.
Hoshi Y, Uchida Y, Tachikawa M, Inoue T, Ohtsuki S, Terasaki T. Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci. 2013;102(9):3343–55.
Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117(2):333–45.
Ohtsuki S, Yamaguchi H, Kang YS, Hori S, Terasaki T. Reduction of L-type amino acid transporter 1 mRNA expression in brain capillaries in a mouse model of Parkinson's disease. Biol Pharm Bull. 2010;33(7):1250–2.
Tarlungeanu DC, Deliu E, Dotter CP, Kara M, Janiesch PC, Scalise M, et al. Impaired amino acid transport at the blood brain barrier is a cause of autism Spectrum disorder. Cell. 2016;167(6):1481–94 e1418.
Salisbury TB, Arthur S. The Regulation and Function of the L-Type Amino Acid Transporter 1 (LAT1) in Cancer. Int J Mol Sci. 2018;19(8).
Zhao Y, Wang L, Pan J. The role of L-type amino acid transporter 1 in human tumors. Intractable Rare Dis Res. 2015;4(4):165–9.
Hafliger P, Charles RP. The L-Type Amino Acid Transporter LAT1-An Emerging Target in Cancer. Int J Mol Sci. 2019;20(10).
Hayashi K, Anzai N. Novel therapeutic approaches targeting L-type amino acid transporters for cancer treatment. World J Gastrointest Oncol. 2017;9(1):21–9.
Cibrian D, Castillo-Gonzalez R, Fernandez-Gallego N, de la Fuente H, Jorge I, Saiz ML, et al. Targeting L-type amino acid transporter 1 in innate and adaptive T cells efficiently controls skin inflammation. J Allergy Clin Immunol. 2020;145(1):199–214 e111.
Alexander GM, Schwartzman RJ, Grothusen JR, Gordon SW. Effect of plasma levels of large neutral amino acids and degree of parkinsonism on the blood-to-brain transport of levodopa in naive and MPTP parkinsonian monkeys. Neurology. 1994;44(8):1491–9.
Cucca A, Mazzucco S, Bursomanno A, Antonutti L, Di Girolamo FG, Pizzolato G, et al. Amino acid supplementation in l-dopa treated Parkinson's disease patients. Clin Nutr. 2015;34(6):1189–94.
Augustyn E, Finke K, Zur AA, Hansen L, Heeren N, Chien HC, et al. LAT-1 activity of meta-substituted phenylalanine and tyrosine analogs. Bioorg Med Chem Lett. 2016;26(11):2616–21.
Nagamori S, Wiriyasermkul P, Okuda S, Kojima N, Hari Y, Kiyonaka S, et al. Structure-activity relations of leucine derivatives reveal critical moieties for cellular uptake and activation of mTORC1-mediated signaling. Amino Acids. 2016;48(4):1045–58.
Ylikangas H, Malmioja K, Peura L, Gynther M, Nwachukwu EO, Leppanen J, et al. Quantitative insight into the design of compounds recognized by the L-type amino acid transporter 1 (LAT1). ChemMedChem. 2014;9(12):2699–707.
Ylikangas H, Peura L, Malmioja K, Leppanen J, Laine K, Poso A, et al. Structure-activity relationship study of compounds binding to large amino acid transporter 1 (LAT1) based on pharmacophore modeling and in situ rat brain perfusion. Eur J Pharm Sci. 2013;48(3):523–31.
Zur AA, Chien HC, Augustyn E, Flint A, Heeren N, Finke K, et al. LAT1 activity of carboxylic acid bioisosteres: evaluation of hydroxamic acids as substrates. Bioorg Med Chem Lett. 2016;26(20):5000–6.
Puris E, Gynther M, Huttunen J, Auriola S, Huttunen KM. L-type amino acid transporter 1 utilizing prodrugs of ferulic acid revealed structural features supporting the design of prodrugs for brain delivery. Eur J Pharm Sci. 2019;129:99–109.
Puris E, Gynther M, Huttunen J, Petsalo A, Huttunen KM. L-type amino acid transporter 1 utilizing prodrugs: how to achieve effective brain delivery and low systemic exposure of drugs. J Control Release. 2017;261:93–104.
Chien HC, Colas C, Finke K, Springer S, Stoner L, Zur AA, et al. Reevaluating the substrate specificity of the L-type amino acid transporter (LAT1). J Med Chem. 2018;61(16):7358–73.
Dickens D, Webb SD, Antonyuk S, Giannoudis A, Owen A, Radisch S, et al. Transport of gabapentin by LAT1 (SLC7A5). Biochem Pharmacol. 2013;85(11):1672–83.
van Bree JB, Audus KL, Borchardt RT. Carrier-mediated transport of baclofen across monolayers of bovine brain endothelial cells in primary culture. Pharm Res. 1988;5(6):369–71.
Gynther M, Jalkanen A, Lehtonen M, Forsberg M, Laine K, Ropponen J, et al. Brain uptake of ketoprofen-lysine prodrug in rats. Int J Pharm. 2010;399(1–2):121–8.
Gynther M, Laine K, Ropponen J, Leppanen J, Mannila A, Nevalainen T, et al. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J Med Chem. 2008;51(4):932–6.
Killian DM, Hermeling S, Chikhale PJ. Targeting the cerebrovascular large neutral amino acid transporter (LAT1) isoform using a novel disulfide-based brain drug delivery system. Drug Deliv. 2007;14(1):25–31.
Peura L, Malmioja K, Huttunen K, Leppanen J, Hamalainen M, Forsberg MM, et al. Design, synthesis and brain uptake of LAT1-targeted amino acid prodrugs of dopamine. Pharm Res. 2013;30(10):2523–37.
Peura L, Malmioja K, Laine K, Leppanen J, Gynther M, Isotalo A, et al. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol Pharm. 2011;8(5):1857–66.
Huttunen KM, Huttunen J, Aufderhaar I, Gynther M, Denny WA, Spicer JA. L-type amino acid transporter 1 (lat1)-mediated targeted delivery of perforin inhibitors. Int J Pharm. 2016;498(1–2):205–16.
Walker I, Nicholls D, Irwin WJ, Freeman S. Drug delivery via active transport at the blood-brain barrier: affinity of a prodrug of phosphonoformate for the large amino acid transporter. Int J Pharm. 1994;104(2):157–67.
Bonina FP, Arenare L, Palagiano F, Saija A, Nava F, Trombetta D, et al. Synthesis, stability, and pharmacological evaluation of nipecotic acid prodrugs. J Pharm Sci. 1999;88(5):561–7.
Balakrishnan A, Jain-Vakkalagadda B, Yang C, Pal D, Mitra AK. Carrier mediated uptake of L-tyrosine and its competitive inhibition by model tyrosine linked compounds in a rabbit corneal cell line (SIRC)--strategy for the design of transporter/receptor targeted prodrugs. Int J Pharm. 2002;247(1–2):115–25.
Gynther M, Peura L, Vernerova M, Leppanen J, Karkkainen J, Lehtonen M, et al. Amino acid Promoieties Alter Valproic acid pharmacokinetics and enable extended brain exposure. Neurochem Res. 2016;41(10):2797–809.
Gynther M, Pickering DS, Spicer JA, Denny WA, Huttunen KM. Systemic and brain pharmacokinetics of Perforin inhibitor Prodrugs. Mol Pharm. 2016;13(7):2484–91.
Huttunen KM, Huttunen J, Aufderhaar I, Gynther M, Denny WA, Spicer JA. L-Type amino acid transporter 1 (lat1)-mediated targeted delivery of perforin inhibitors. Int J Pharm. 2016;498(1–2):205–16 73.
Puris E, Gynther M, de Lange ECM, Auriola S, Hammarlund-Udenaes M, Huttunen KM, et al. Mechanistic Study on the Use of the l-Type Amino Acid Transporter 1 for Brain Intracellular Delivery of Ketoprofen via Prodrug: A Novel Approach Supporting the Development of Prodrugs for Intracellular Targets. Mol Pharm. 2019;16(7):3261–74.
Huttunen J, Peltokangas S, Gynther M, Natunen T, Hiltunen M, Auriola S, et al. L-type amino acid transporter 1 (LAT1/Lat1)-utilizing Prodrugs can improve the delivery of drugs into neurons. Astrocytes and Microglia Sci Rep. 2019;9(1):12860.
Huttunen KM. Identification of human, rat and mouse hydrolyzing enzymes bioconverting amino acid ester prodrug of ketoprofen. Bioorg Chem. 2018;81:494–503.
Atluri H, Talluri RS, Mitra AK. Functional activity of a large neutral amino acid transporter (LAT) in rabbit retina: a study involving the in vivo retinal uptake and vitreal pharmacokinetics of L-phenyl alanine. Int J Pharm. 2008;347(1–2):23–30.
Usui T, Kubo Y, Akanuma S, Hosoya K. Beta-alanine and l-histidine transport across the inner blood-retinal barrier: potential involvement in L-carnosine supply. Exp Eye Res. 2013;113:135–42.
Katragadda S, Gunda S, Hariharan S, Mitra AK. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: evaluation of their utility in treating ocular HSV infections. Int J Pharm. 2008;359(1–2):15–24.
Suresh K, Xiadong Z, Ravi TS, Mitra AK. Small neutral amino acid Ester Prodrugs of acyclovir targeting amino acid transporters on the cornea: possible antiviral agents against ocular HSV-1 infections. Ophthalmol Eye Dis. 2010;2:43–56.
Akanuma SI, Yamakoshi A, Sugouchi T, Kubo Y, Hartz AMS, Bauer B, et al. Role of l-type amino acid transporter 1 at the inner blood-retinal barrier in the blood-to-retina transport of gabapentin. Mol Pharm. 2018;15(6):2327–37.
Shennan DB, Thomson J, Gow IF, Travers MT, Barber MC. L-leucine transport in human breast cancer cells (MCF-7 and MDA-MB-231): kinetics, regulation by estrogen and molecular identity of the transporter. Biochim Biophys Acta. 2004;1664(2):206–16.
Kim DK, Kanai Y, Choi HW, Tangtrongsup S, Chairoungdua A, Babu E, et al. Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta. 2002;1565(1):112–21.
Lin J, Raoof DA, Thomas DG, Greenson JK, Giordano TJ, Robinson GS, et al. L-type amino acid transporter-1 overexpression and melphalan sensitivity in Barrett's adenocarcinoma. Neoplasia. 2004;6(1):74–84.
Greig NH, Momma S, Sweeney DJ, Smith QR, Rapoport SI. Facilitated transport of melphalan at the rat blood-brain barrier by the large neutral amino acid carrier system. Cancer Res. 1987;47(6):1571–6.
Hosoya K, Kyoko H, Toyooka N, Kato A, Orihashi M, Tomi M, et al. Evaluation of amino acid-mustard transport as L-type amino acid transporter 1 (LAT1)-mediated alkylating agents. Biol Pharm Bull. 2008;31(11):2126–30.
Haines DR, Fuller RW, Ahmad S, Vistica DT, Marquez VE. Selective cytotoxicity of a system L specific amino acid nitrogen mustard. J Med Chem. 1987;30(3):542–7.
Takada Y, Greig NH, Vistica DT, Rapoport SI, Smith QR. Affinity of antineoplastic amino acid drugs for the large neutral amino acid transporter of the blood-brain barrier. Cancer Chemother Pharmacol. 1991;29(2):89–94.
Matharu J, Oki J, Worthen DR, Smith QR, Crooks PA. Regiospecific and conformationally restrained analogs of melphalan and DL-2-NAM-7 and their affinities for the large neutral amino acid transporter (system LAT1) of the blood-brain barrier. Bioorg Med Chem Lett. 2010;20(12):3688–91.
Williams MG, Earhart RH, Bailey H, McGovren JP. Prevention of central nervous system toxicity of the antitumor antibiotic acivicin by concomitant infusion of an amino acid mixture. Cancer Res. 1990;50(17):5475–80.
Hong S, Fang Z, Jung HY, Yoon JH, Hong SS, Maeng HJ. Synthesis of Gemcitabine-Threonine Amide Prodrug Effective on Pancreatic Cancer Cells with Improved Pharmacokinetic Properties. Molecules. 2018;23(10).
Wu W, Dong Y, Gao J, Gong M, Zhang X, Kong W, et al. Aspartate-modified doxorubicin on its N-terminal increases drug accumulation in LAT1-overexpressing tumors. Cancer Sci. 2015;106(6):747–56.
Singh VK, Subudhi BB. Development and characterization of lysine-methotrexate conjugate for enhanced brain delivery. Drug Deliv. 2016;23(7):2327–37.
An S, Lu X, Zhao W, Sun T, Zhang Y, Lu Y, et al. Amino acid metabolism abnormity and microenvironment variation mediated targeting and controlled Glioma chemotherapy. Small. 2016;12(40):5633–45.
Bhunia S, Vangala V, Bhattacharya D, Ravuri HG, Kuncha M, Chakravarty S, et al. Large amino acid transporter 1 selective liposomes of l-DOPA functionalized Amphiphile for combating Glioblastoma. Mol Pharm. 2017;14(11):3834–47.
Li L, Di X, Wu M, Sun Z, Zhong L, Wang Y, et al. Targeting tumor highly-expressed LAT1 transporter with amino acid-modified nanoparticles: toward a novel active targeting strategy in breast cancer therapy. Nanomedicine. 2017;13(3):987–98.
Li L, Di X, Zhang S, Kan Q, Liu H, Lu T, et al. Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf B Biointerfaces. 2016;141:260–7.
Ong ZY, Chen S, Nabavi E, Regoutz A, Payne DJ, Elson DS, et al. Multibranched gold nanoparticles with intrinsic LAT-1 targeting capabilities for selective Photothermal therapy of breast Cancer. ACS Appl Mater Interfaces. 2017;9(45):39259–70.
Langstrom B, Antoni G, Gullberg P, Halldin C, Malmborg P, Nagren K, et al. Synthesis of L- and D-[methyl-11C]methionine. J Nucl Med. 1987;28(6):1037–40.
Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40(4):615–35.
Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neuro-Oncol. 2010;99(2):217–25.
Grosu AL, Astner ST, Riedel E, Nieder C, Wiedenmann N, Heinemann F, et al. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C] methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys. 2011;81(4):1049–58.
Lahoutte T, Caveliers V, Camargo SM, Franca R, Ramadan T, Veljkovic E, et al. SPECT and PET amino acid tracer influx via system L (h4F2hc-hLAT1) and its transstimulation. J Nucl Med. 2004;45(9):1591–6.
Habermeier A, Graf J, Sandhofer BF, Boissel JP, Roesch F, Closs EI. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids. 2015;47(2):335–44.
Becherer A, Karanikas G, Szabo M, Zettinig G, Asenbaum S, Marosi C, et al. Brain tumour imaging with PET: a comparison between [18F] fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003;30(11):1561–7.
Lee SW, Shim SR, Jeong SY, Kim SJ. Comparison of 5 different PET radiopharmaceuticals for the detection of recurrent medullary thyroid carcinoma: a network Meta-analysis. Clin Nucl Med. 2020;45:341–8.
Youland RS, Kitange GJ, Peterson TE, Pafundi DH, Ramiscal JA, Pokorny JL, Giannini C, Laack NN, Parney IF, Lowe VJ, Brinkmann DH, Sarkaria JN. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neuro-Oncol 2013;111(1):11–18.
Tomiyoshi K, Amed K, Muhammad S, Higuchi T, Inoue T, Endo K, et al. Synthesis of isomers of 18F-labelled amino acid radiopharmaceutical: position 2- and 3-L-18F-alpha-methyltyrosine using a separation and purification system. Nucl Med Commun. 1997;18(2):169–75.
Wiriyasermkul P, Nagamori S, Tominaga H, Oriuchi N, Kaira K, Nakao H, et al. Transport of 3-fluoro-L-alpha-methyl-tyrosine by tumor-upregulated L-type amino acid transporter 1: a cause of the tumor uptake in PET. J Nucl Med. 2012;53(8):1253–61.
Kaira K, Oriuchi N, Otani Y, Shimizu K, Tanaka S, Imai H, et al. Fluorine-18-alpha-methyltyrosine positron emission tomography for diagnosis and staging of lung cancer: a clinicopathologic study. Clin Cancer Res. 2007;13(21):6369–78.
Nobusawa A, Kim M, Kaira K, Miyashita G, Negishi A, Oriuchi N, et al. Diagnostic usefulness of (1)(8)F-FAMT PET and L-type amino acid transporter 1 (LAT1) expression in oral squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2013;40(11):1692–700.
Nozaki S, Nakatani Y, Mawatari A, Shibata N, Hume WE, Hayashinaka E, et al. (18)F-FIMP: a LAT1-specific PET probe for discrimination between tumor tissue and inflammation. Sci Rep. 2019;9(1):15718.
Kankaanranta L, Saarilahti K, Makitie A, Valimaki P, Tenhunen M, Joensuu H. Boron neutron capture therapy (BNCT) followed by intensity modulated chemoradiotherapy as primary treatment of large head and neck cancer with intracranial involvement. Radiother Oncol. 2011;99(1):98–9.
Kawabata S, Miyatake S, Kuroiwa T, Yokoyama K, Doi A, Iida K, et al. Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res. 2009;50(1):51–60.
Barth RF, Coderre JA, Vicente MG, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11(11):3987–4002.
Wongthai P, Hagiwara K, Miyoshi Y, Wiriyasermkul P, Wei L, Ohgaki R, et al. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015;106(3):279–86.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank Dr. Ewen MacDonald for language editing. The study was supported by the Academy of Finland (K.M.H., grant numbers 294227 and 307057), Finnish Cultural Foundation (E.P.), Alfred Kordelin Foundation (E.P.) and the University of Eastern Finland Doctoral School.
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Puris, E., Gynther, M., Auriola, S. et al. L-Type amino acid transporter 1 as a target for drug delivery. Pharm Res 37, 88 (2020). https://doi.org/10.1007/s11095-020-02826-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02826-8