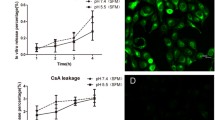
Abstract
Cequa®, a unique and first-in-class preservative free cyclosporine-A (CsA) nanomicellar topical formulation was recently approved by US FDA for treatment of dry eye disease or keratoconjuntivitis sicca (KCS). Being highly hydrophobic, CsA is currently available as an oil based emulsion, which has its own shortcomings. Developing an aqueous and clear formulation of CsA is imperative yet a challenging need in the quest for a safe and better drug product. In this regard, a novel, clear, aqueous nanomicellar solution of CsA was developed which has the potential to deliver therapeutic concentrations of CsA with minimal discomfort to patients. Highly promising pre-clinical results of Cequa® (OTX-101), has led to its advancement to the clinical trials. Phase III clinical trials have demonstrated that OTX-101 is highly effective, safe, and has a rapid onset of action in treating KCS. This review presents a comprehensive insight on formulation development, preclinical and clinical pharmacokinetic results of Cequa®. Additionally, the translational development of Cequa® from the laboratory benchtop to patient bedside has been discussed.













Similar content being viewed by others
Abbreviations
- APCs:
-
Antigen presenting cells
- AUC:
-
Area under curve
- BSS:
-
Balanced salt solution
- CaN:
-
Calcineurin
- CAPIR:
-
Circulation, accumulation, penetration, internalization and release
- CMC:
-
Critical micelle concentration
- CsA:
-
Cyclosporine
- FK:
-
Filamentary keratitis
- ICAM-1:
-
Intercellular adhesion molecule 1
- IC:
-
Impression cytology
- IL-2:
-
Interleukin 2
- IOP:
-
Intraocular pressure
- KCS:
-
Keratoconjunctivitis sicca
- LFU:
-
Lacrimal functional unit
- MGD:
-
Meibomian gland dysfunction
- MMP-9:
-
Matrix metallopeptidase 9
- MPTP:
-
Mitochondrial permeability transition pore
- NF-ATc:
-
Cytoplasmic component of nuclear factor of activated T cells
- NF-ATn:
-
Nuclear component of nuclear factor of activated T cells
- PEG:
-
Polyethylene glycol
- PK:
-
Pharmacokinetics
- VCAM-1:
-
Vascular cell adhesion molecule-1
References
Javadi MA, Feizi S. Dry eye syndrome. J Ophthalmic Vis Res. 2011;6(3):192–8.
Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–12.
Hawkes N. US's $2bn annual spend on dry eye disease "brings tears to your eyes," say critics. BMJ. 2018;360:k492.
Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379–87.
Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998;17(4):565–96.
Butovich IA. Tear film lipids. Exp Eye Res. 2013;117:4–27.
Cwiklik L. Tear film lipid layer: a molecular level view. Biochim Biophys Acta. 2016;1858(10):2421–30.
Zhang X, VJ M, Qu Y, He X, Ou S, Bu J, et al. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int J Mol Sci. 2017;18(7).
Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17(6):584–9.
Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). The ocular surface. 2007;5(2):179–193.
Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81 quiz 82.
Reyes JL, Vannan DT, Eksteen B, Avelar IJ, Rodriguez T, Gonzalez MI, et al. Innate and adaptive cell populations driving inflammation in dry eye disease. Mediat Inflamm. 2018;2018:2532314.
Kuklinski E, Asbell PA. Sjogren's syndrome from the perspective of ophthalmology. Clin Immunol. 2017;182:55–61.
Perry HD. Dry eye disease: pathophysiology, classification, and diagnosis. Am J Manag Care. 2008;14(3 Suppl):S79–87.
Sullivan DA, Dana R, Sullivan RM, Krenzer KL, Sahin A, Arica B, et al. Meibomian gland dysfunction in primary and secondary Sjogren syndrome. Ophthalmic Res. 2018;59(4):193–205.
Milner MS, Beckman KA, Luchs JI, Allen QB, Awdeh RM, Berdahl J, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27(Suppl 1):3–47.
Roy NS, Wei Y, Kuklinski E, Asbell PA. The growing need for validated biomarkers and endpoints for dry eye clinical research. Invest Ophthalmol Vis Sci. 2017;58(6):BIO1–BIO19.
Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123(11):2300–8.
Buckley RJ. Assessment and management of dry eye disease. Eye. 2018;32(2):200–3.
Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21(4):310–6.
Levy O, Labbe A, Borderie V, Laroche L, Bouheraoua N. Topical cyclosporine in ophthalmology: pharmacology and clinical indications. J Fr Ophtalmol. 2016;39(3):292–307.
Schultz C. Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye Dis. 2014;6:37–42.
Leonardi A, Flamion B, Baudouin C. Keratitis in dry eye disease and topical Ciclosporin a. Ocul Immunol Inflamm. 2017;25(4):577–86.
Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin a. Ocul Immunol Inflamm. 2010;18(5):352–61.
Boboridis KG, Konstas AGP. Evaluating the novel application of cyclosporine 0.1% in ocular surface disease. Expert Opin Pharmacother. 2018;19(9):1027–39.
Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2–3):119–25.
Pietro DCaAD. Systemic Cyclosporin in the treatment of psoriasis. IntechOpen 2012.
Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA phase 3 study group. Ophthalmology. 2000;107(4):631–9.
Sy A, O'Brien KS, Liu MP, Cuddapah PA, Acharya NR, Lietman TM, et al. Expert opinion in the management of aqueous deficient dry eye disease (DED). BMC Ophthalmol. 2015;15:133.
Bell TA, Hunnisett AG. Cyclosporin a: tissue levels following topical and systemic administration to rabbits. Br J Ophthalmol. 1986;70(11):852–5.
Pfau B, Kruse FE, Rohrschneider K, Zorn M, Fiehn W, Burk RO, et al. Comparison between local and systemic administration of cyclosporin a on the effective level in conjunctiva, aqueous humor and serum. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 1995;92(6):833–9.
Ganesan V, Milford DV, Taylor CM, Hulton SA, Parvaresh S, Ramani P. Cyclosporin-related nephrotoxicity in children with nephrotic syndrome. Pediatr Nephrol. 2002;17(3):225–6 author reply 227.
el-Asrar AM, Tabbara KF, Geboes K, Missotten L, Desmet V. An immunohistochemical study of topical cyclosporine in vernal keratoconjunctivitis. Am J Ophthalmol. 1996;121(2):156–61.
Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–8.
Parrilha LR, Nai GA, Giuffrida R, Barbero RC, Padovani LD, Pereira RH, et al. Comparison of 1% cyclosporine eye drops in olive oil and in linseed oil to treat experimentally-induced keratoconjunctivitis sicca in rabbits. Arq Bras Oftalmol. 2015;78(5):295–9.
Benitez del Castillo JM, del Aguila C, Duran S, Hernandez J, Garcia Sanchez J. Influence of topically applied cyclosporine a in olive oil on corneal epithelium permeability. Cornea. 1994;13(2):136–40.
Williams DL. A comparative approach to topical cyclosporine therapy. Eye. 1997;11(Pt 4):453–64.
Agarwal P, Rupenthal ID. Modern approaches to the ocular delivery of cyclosporine a. Drug Discov Today. 2016;21(6):977–88.
Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin a ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin a phase 2 study group. Ophthalmology. 2000;107(5):967–74.
Prabhu SS, Shtein RM, Michelotti MM, Cooney TM. Topical cyclosporine a 0.05% for recurrent anterior uveitis. Br J Ophthalmol. 2016;100(3):345–7.
Rhee MK, Mah FS. Clinical utility of cyclosporine (CsA) ophthalmic emulsion 0.05% for symptomatic relief in people with chronic dry eye: a review of the literature. Clin Ophthalmol. 2017;11:1157–66.
Hoy SM. Ciclosporin ophthalmic emulsion 0.1%: a review in severe dry eye disease. Drugs. 2017;77(17):1909–16.
Boujnah Y, Mouchel R, El-Chehab H, Dot C, Burillon C, Kocaba V. Prospective, monocentric, uncontrolled study of efficacy, tolerance and adherence of cyclosporin 0.1% for severe dry eye syndrome. J Fr Ophtalmol. 2018;41(2):129–35.
Ames P, Galor A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidence. Clinical investigation. 2015;5(3):267–85.
Baudouin C, de la Maza MS, Amrane M, Garrigue JS, Ismail D, Figueiredo FC, Leonardi A. One-year efficacy and safety of 0.1% cyclosporine a cationic emulsion in the treatment of severe dry eye disease. Eur J Ophthalmol 2017:0.
Stonecipher KG, Chia J, Onyenwenyi A, Villanueva L, Hollander DA. Health claims database study of cyclosporine ophthalmic emulsion treatment patterns in dry eye patients. Ther Clin Risk Manag. 2013;9:409–15.
Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine a delivery to the eye: a comprehensive review of academic and industrial efforts. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2017;117:14–28.
Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(5):422–37.
Kamaleddin MA. Nano-ophthalmology: applications and considerations. Nanomedicine. 2017;13(4):1459–72.
Irfan M, Usman M, Mansha A, Rasool N, Ibrahim M, Rana UA, et al. Thermodynamic and spectroscopic investigation of interactions between reactive red 223 and reactive orange 122 anionic dyes and cetyltrimethyl ammonium bromide (CTAB) cationic surfactant in aqueous solution. TheScientificWorldJOURNAL. 2014;2014:540975.
Tanford C. Thermodynamics of micelle formation: prediction of micelle size and size distribution. Proc Natl Acad Sci U S A. 1974;71(5):1811–5.
Mandal A, Cholkar K, Khurana V, Shah A, Agrahari V, Bisht R, et al. Topical formulation of self-assembled antiviral prodrug Nanomicelles for targeted retinal delivery. Mol Pharm. 2017;14(6):2056–69.
Lu Y, Yue Z, Xie J, Wang W, Zhu H, Zhang E, et al. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat Biomed Eng. 2018;2(5):318–25.
Morishima K, Sugawara S, Yoshimura T, Shibayama M. Structure and rheology of wormlike micelles formed by fluorocarbon-hydrocarbon-type hybrid Gemini surfactant in aqueous solution. Langmuir. 2017;33(24):6084–91.
Dhakal S, Sureshkumar R. Anomalous diffusion and stress relaxation in surfactant micelles. Phys Rev E. 2017;96(1–1):012605.
Paradies HH. Shape and size of a nonionic surfactant micelle. Triton X-100 in aqueous solution. J Phys Chem. 1980;84(6):599–607.
Dill KA, Flory PJ. Molecular organization in micelles and vesicles. Proc Natl Acad Sci. 1981;78(2):676–80.
Ahn YN, Mohan G, Kopelevich DI. Collective degrees of freedom involved in absorption and desorption of surfactant molecules in spherical non-ionic micelles. J Chem Phys. 2012;137(16):164902.
Cholkar K, Gilger BC, Mitra AK. Topical, aqueous, clear cyclosporine formulation Design for Anterior and Posterior Ocular Delivery. Transl Vis Sci Technol. 2015;4(3):1.
Lasic DD. Mixed micelles in drug delivery. Nature. 1992;355:279–80.
Alexander-Bryant AA, Vanden Berg-Foels WS, Wen X. Bioengineering strategies for designing targeted cancer therapies. Adv Cancer Res. 2013;118:1–59.
Kim SA, Jeong KJ, Yethiraj A, Mahanthappa MK. Low-symmetry sphere packings of simple surfactant micelles induced by ionic sphericity. Proc Natl Acad Sci U S A. 2017;114(16):4072–7.
Xotta G, Mazzucco G, Salomoni VA, Majorana CE, Willam KJ. Composite behavior of concrete materials under high temperatures. Int J Solids Struct. 2015;64–65:86–99.
Lipfert J, Columbus L, Chu VB, Lesley SA, Doniach S. Size and shape of detergent micelles determined by small-angle X-ray scattering. J Phys Chem B. 2007;111(43):12427–38.
Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc Natl Acad Sci U S A. 2008;105(18):6596–601.
Wang J, Mao W, Lock LL, Tang J, Sui M, Sun W, et al. The role of micelle size in tumor accumulation, penetration, and treatment. ACS Nano. 2015;9(7):7195–206.
Chopra P, Hao J, Li SK. Iontophoretic transport of charged macromolecules across human sclera. Int J Pharm. 2010;388(1–2):107–13.
Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32(8):2244–58.
Motolko M, Breslin CW. The effect of pH and osmolarity on the ability of tolerate artificial tears. Am J Ophthalmol. 1981;91(6):781–4.
Suknuntha K, Tantishaiyakul V, Worakul N, Taweepreda W. Characterization of muco- and bioadhesive properties of chitosan, PVP, and chitosan/PVP blends and release of amoxicillin from alginate beads coated with chitosan/PVP. Drug Dev Ind Pharm. 2011;37(4):408–18.
Cholkar K, Gilger BC, Mitra AK. Topical delivery of aqueous micellar resolvin E1 analog (RX-10045). Int J Pharm. 2016;498(1–2):326–34.
Ashim K. Mitra SLW, Eugene J. McNally topical formulations and uses thereof. In. USA: Sun Pharma Global FZE; 2015.
Mandal A, Pal D, Agrahari V, Trinh HM, Joseph M, Mitra AK. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv Drug Deliv Rev. 2018;126:67–95.
Mandal A, Bisht R, Rupenthal ID, Mitra AK. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. 2017;248:96–116.
Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57(14):2010–32.
Hernandez C, Garcia-Ramirez M, Corraliza L, Fernandez-Carneado J, Farrera-Sinfreu J, Ponsati B, et al. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2013;62(7):2569–78.
Schoenwald RD, Deshpande GS, Rethwisch DG, Barfknecht CF. Penetration into the anterior chamber via the conjunctival/scleral pathway. J Ocul Pharmacol Ther. 1997;13(1):41–59.
Maurice DM. Drug delivery to the posterior segment from drops. Surv Ophthalmol. 2002;47(Suppl 1):S41–52.
Cholkar K. Topical clear aqueous Nanomicellar formulations for anterior and posterior ocular drug delivery. In. Kansas City University of Missouri – Kansas City; 2015.
Luschmann C, Herrmann W, Strauss O, Luschmann K, Goepferich A. Ocular delivery systems for poorly soluble drugs: an in-vivo evaluation. Int J Pharm. 2013;455(1–2):331–7.
Ashim K. Mitra PRV, Ulrich M. Grau Topical Drug Delivery Systems For Ophthalmic Use. In.: Aurinia Pharmaceuticals Inc Apr. 28, 2015.
Acheampong AA, Shackleton M, Tang-Liu DD, Ding S, Stern ME, Decker R. Distribution of cyclosporin a in ocular tissues after topical administration to albino rabbits and beagle dogs. Curr Eye Res. 1999;18(2):91–103.
Schwartz LM, Woloshin S. A clear-eyed view of Restasis and chronic dry eye disease. JAMA Intern Med. 2018;178(2):181–2.
Gilger SLWWKPVBC. Ocular distribution of cyclosporine following topical administration of OTX-101 in New Zealand white rabbits. In.The Association for Research in Vision and Ophthalmology. Honolulu, Hawaii; 2018.
Tauber J, Schechter BA, Bacharach J, Toyos MM, Smyth-Medina R, Weiss SL, et al. A phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–9.
Sun Pharma Announces U.S. FDA Approval of CEQUA™ to Treat Dry Eye Disease. Sun Pharmaceutical Industries Ltd.; Available from: https://www.businesswire.com/news/home/20180815005765/en/Sun-Pharma-Announces-U.S.-FDA-Approval-CEQUA%E2%84%A2. Accessed 29 Nov 2018.
Sheppard JD, Torkildsen GL, Lonsdale JD, D'Ambrosio FA Jr, McLaurin EB, Eiferman RA, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121(2):475–83.
Tauber J, Karpecki P, Latkany R, Luchs J, Martel J, Sall K, et al. Investigators O-. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 study. Ophthalmology. 2015;122(12):2423–31.
Jodi Luchs M. Phase 3 Clinical Results of Cyclosporine 0.09% in a New Nanomicellar Ophthalmic Solution to Treatment Keratoconjunctivitis Sicca. In.American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting Washington, DC; 2018.
Lifitegrast (Xiidra) for Dry Eye Disease. Jama. 2017;317(14):1473–1474.
Kim HS, Kim TI, Kim JH, Yoon KC, Hyon JY, Shin KU, et al. Evaluation of clinical efficacy and safety of a novel Cyclosporin a Nanoemulsion in the treatment of dry eye syndrome. J Ocul Pharmacol Ther. 2017;33(7):530–8.
Schultz C. Voclosporin as a treatment for noninfectious uveitis. Ophthalmol Eye Dis. 2013;5:5–10.
Abidi A, Shukla P, Ahmad A. Lifitegrast: a novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194–8.
Acknowledgements and Disclosures
The authors would like to acknowledge the contributions of Dr. Kishore Cholkar and Dr. Brian C. Gilger for the pharmacokinetic portions of the pre-clinical studies. The authors also acknowledge Joseph Tauber (Tauber Eye Centre), Sidney L. Weiss (Auven Therapeutics), William Kramer (Kramer consulting LLC) and Poonam Velagaleti (I-novion, Inc.) for their contributions in the various phases of the clinical studies. Authors also acknowledge Ocular Technologies Sarl (now wholly owned subsidiary of Sun Pharmaceutical Industries) and Sun Pharmaceutical Industries for sponsoring, conducting, monitoring and analyzing the clinical studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Hovhannes J Gukasyan, Shumet Hailu, and Thomas Karami
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Mandal, A., Gote, V., Pal, D. et al. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm Res 36, 36 (2019). https://doi.org/10.1007/s11095-018-2556-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2556-5