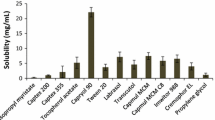
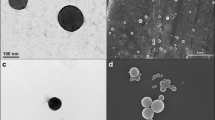
Abstract
Purpose
Oral drug delivery using NPs is a current strategy for poorly absorbed molecules. It offers significant improvement in terms of bioavailability. However, the encapsulation of proteins and peptides in polymeric NPs is a challenge. Firstly, the present study focused on the double emulsion process in order to encapsulate the OXY peptide. Then the technique was challenged by a one-step simplified process, the simple emulsion.
Methods
In order to study the influence of formulation and process parameters, factorial experimental designs were carried on. The responses observed were the NP size (<200 nm in order to penetrate the intestinal mucus layer), the suspension stability (ZP < |30| mV) and the OXY loading.
Results
It was thus found that the amount and the nature of surfactant, the ratio between the phases, the amount of PLA-PEG polymer and OXY, the presence of a viscosifying agent, and the duration of the sonication could significantly influence the responses. Finally, OXY-loaded NPs from both processes were obtained with NP size of 195 and 226 nm and OXY loading of 4 and 3.3% for double and simple emulsions, respectively.
Conclusion
The two processes appeared to be suitable for OXY encapsulation and comparable in term of NP size, peptide drug load and release obtained.





Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- ATFA:
-
Trifluoroacetic acid
- BSA:
-
Bovine serum albumin
- DCM:
-
Dichloromethane
- DL:
-
Drug load
- DLS:
-
Dynamic light scattering
- DMSO :
-
Dimethyl sulfoxyde
- EtAc:
-
Ethyl acetate
- EtOH:
-
Ethanol
- HBSS:
-
Hanks buffer saline solution
- HEC:
-
Hydroxy ethyl cellulose
- HLB:
-
Hydrophilic-lipophilic balance
- HPLC:
-
High pressure liquid chromatography
- MES:
-
2-(N-morpholino)ethanesulfonic acid
- NME:
-
New molecular entity
- NP:
-
Nanoparticle
- O:
-
Organic phase
- OXY:
-
Oxytocin
- PDLG:
-
DL-lactide/glycolide copolymer
- PEG:
-
Poly-ethylene glycol
- PLA:
-
Polylactic acid
- PLGA:
-
Poly-D-L-lactide-co-glycolide
- PVA:
-
Poly(vinyl alcohol)
- W:
-
Aqueous phase
- WE:
-
External aqueous phase
- WI:
-
Internal aqueous phase
- ZP:
-
Zeta potential
References
Reis CP, Neufeld RJ, Ribeiro AJ. VeigaF. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine. 2006;2:8–21.
Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. Journal of Nanobiotechnology. 2011;9:55.
Damgé C, Michel C, Aprahamian M, Couvreur P, Devissaguet JP. Nanocapsules as carriers for oral peptide delivery. J Control Release. 1990;13:233–9.
Iqbal M, Zafar N, Fessi H, Elaissari A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int J Pharm. 2015;496:173–90.
Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26:1025–58.
Wang H, Zhao Y, Wu Y, Hu YL, Nan K, Nie G, et al. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32:8281–90.
Mattos AC, Altmeyer C, Tominaga TT, Khalil NM, Mainardes RM. Polymeric nanoparticles for oral delivery of 5-fluorouracil: formulation optimization, cytotoxicity assay and pre-clinical pharmacokinetics study. Eur J Pharm Sci. 2016;84:83–91.
Danafar H, Rostamizadeh K, Davaran S, Hamidi M. Drug-conjugated PLA-PEG-PLA copolymers: a novel approach for controlled delivery of hydrophilic drugs by micelle formation. Pharm Dev Technol. 2017;22(8):947–57.
Tomar L, Tyagi C, Kumar M, Kumar P, Singh H, Choonara YE, et al. In vivo evaluation of a conjugated poly(lactide-ethylene glycol) nanoparticle depot formulation for prolonged insulin delivery in the diabetic rabbit model. Int J Nanomedicine. 2013;8:505–20.
Jain A, Jain SK. L-valine appended PLGA nanoparticles for oral insulin delivery. Acta Diabetol. 2015;52:663–76.
Pirooznia N, Hasannia S, Lotfi AS, Ghanei M. Encapsulation of alpha-1 antitrypsin in PLGA nanoparticles: in vitro characterization as an effective aerosol formulation in pulmonary diseases. J Nanobiotechnology. 2012;10:20.
Essa S, Rabanel JM, Hildgen P. Characterization of rhodamine loaded PEG-g-PLA nanoparticles (NPs): effect of poly(ethylene glycol) grafting density. Int J Pharm. 2011;411:178–87.
Ji S, Lu J, Liu Z, Srivastava D, Song A, Liu Y, et al. Dynamic encapsulation of hydrophilic nisin in hydrophobic poly (lactic acid) particles with controlled morphology by a single emulsion process. J Colloid Interface Sci. 2014;423:85–93.
Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–70.
Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opinion Solid State Mater Sci. 2002;6:319–27.
Budhian A, Siegel SJ, Winey KI. Haloperidol-loaded PLGA nanoparticles: systematic study of particle size and drug content. Int J Pharm. 2007;336:367–75.
Görner T, Gref R, Michenot D, Sommer F, Tran MN, Dellacherie E. Lidocaine-loaded biodegradable nanospheres. I. Optimization of the drug incorporation into the polymer matrix. J Control Release. 1999;57:259–68.
Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, et al. Transepithelial transport of fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med. 2013;5:213ra167.
Sharma N, Madan P, Lin S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: a co-surfactant study. Asian Journal of Pharmaceutical Sciences. 2016;11:404–16.
Zweers ML, Grijpma DW, Engbers GH, Feijen J. The preparation of monodisperse biodegradable polyester nanoparticles with a controlled size. J Biomed Mater Res B Appl Biomater. 2003;66:559–66.
Box GEP, Hunter WG, Hunter JS. Statistics for experimenters: an introduction to design, data analysis, and model building. Hoboken: Wiley; 1978.
Julienne MC, Alonso MJ, Gomez Amoza JL, Benoit JP. Preparation of poly(D,L-lactide/glycolide) nanoparticles of controlled particle size distribution: application of experimental designs. Drug DevIndPharm. 1992;18:1063–77.
Lewis GA, Mathieu D, Phan-Tan-Luu R. Pharmaceutical experimental design. New York: Marcel Dekker, Inc.; 1999. p. 186-191
Walls ZF, Gupta SV, Amidon GL, Lee KD. Synthesis and characterization of valyloxy methoxy luciferin for the detection of valacyclovirase and peptide transporter. Bioorg Med Chem Lett. 2014;24:4781–3.
Bilati U, Allemann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75.
Gourdon B, Chemin C, Moreau A, Arnauld T, Baumy P, Cisternino S, et al. Functionalized PLA-PEG nanoparticles targeting intestinal transporter PepT1 for oral delivery of acyclovir. Int J Pharm. 2017;529:357–70.
Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, et al. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–76.
Budhian A, Siegel SJ, Winey KI. Production of haloperidol-loaded PLGA nanoparticles for extended controlled drug release of haloperidol. J Microencapsul. 2005;22:773–85.
Acknowledgments and Disclosures
The author reports no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
Apparent viscosity (Pas) of the viscofying agents HEC at 1% and 2.5% (w/v) and PEG 400 at (3:2) and (2:3) ratios with water, represented as function of shearing speed (1/s), in [water + poloxamer P188 1% (w/v)] aqueous phase (JPEG 83 kb)
Rights and permissions
About this article
Cite this article
Gourdon, B., Declèves, X., Péan, JM. et al. Double or Simple Emulsion Process to Encapsulate Hydrophilic Oxytocin Peptide in PLA-PEG Nanoparticles. Pharm Res 35, 82 (2018). https://doi.org/10.1007/s11095-018-2358-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2358-9