Abstract
Purpose
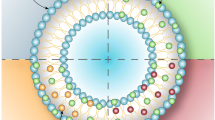
As trisodium L-ascorbyl 2-phosphate 6-palmitate (APPS), an ascorbic acid derivative, is an amphiphilic substance, it forms micelles in aqueous solutions. Micelles are used as drug carriers and can emulsify drugs that are poorly soluble in water, such as nadifloxacin (NDFX). The purpose of this study was to prepare nanocarriers using APPS to carry NDFX into Yucatan micropig skin.
Methods
After synthesis of the NDFX nanoparticles by using the hydration method, physical evaluations were carried out that included assessments of particle size and zeta potential, encapsulation efficiency, particle structure by transmission electron microscopy, 31P–NMR spectra, and particle stability. Functional evaluations of the nanoparticles included 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assays, skin penetration tests, and fluorescence microscopy observations.
Results
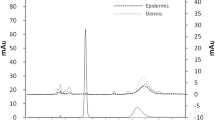
The encapsulation efficiency of NDFX in the nanoparticles was approximately 75%. With added magnesium chloride, the nanoparticles remained stably dispersed in aqueous solution for at least 14 days at 25°C under protection from light. In addition, the nanoparticle formulation improved the skin permeability of NDFX.
Conclusion
APPS-derived nanoparticles were shown to be useful as skin-targeting nanocarriers.










Similar content being viewed by others
Abbreviations
- APPS:
-
Trisodium L-ascorbyl 2-phosphate 6-palmitate
- DDSs:
-
Drug delivery systems
- DLS:
-
Dynamic light scattering
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- DSPE-PEG 2000:
-
Distearoyl phosphatidylethanolamine-polyethylene glycol 2000
- EE%:
-
Encapsulation efficiency rate
- HPLC:
-
High-performance liquid chromatography
- IC50 :
-
50% inhibitory concentration
- IPM:
-
Isopropyl myristate
- NDFX:
-
Nadifloxacin
- PEG:
-
Polyethylene glycol
- TEM:
-
Transmission electron microscopy
- YMP:
-
Yucatan micropig
References
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B: Biointerfaces. 2010;75(1):1–18.
Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P. Biocompatibility of engineered nanoparticles for drug delivery. J Control Release. 2013;166(2):182–94.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: An overview of biomedical applications. J Control Release. 2012;161(2):505–22.
Zhang L, Li Y, Yu JC. Chemical modification of inorganic nanostructures for targeted and controlled drug delivery in cancer treatment. J Mater Chem B. 2014;2(5):452–70.
Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today. 2011;16(7–8):354–60.
Fryd MM, Mason TG. Advanced Nanoemulsions. Annu Rev Phys Chem. 2012;63(1):493–518.
Lohani A, Verma A, Joshi H, Yadav N, Karki N. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014;843687. https://doi.org/10.1155/2014/843687.
Musa SH, Basri M, Masoumi HRF, Shamsudin N, Salim N. Enhancement of physicochemical properties of nanocolloidal carrier loaded with cyclosporine for topical treatment of psoriasis: In vitro diffusion and in vivo hydrating action. Int J Nanomedicine. 2017;12:2427–41.
Ghate VM, Lewis SA, Prabhu P, Dubey A, Patel N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur J Pharm Biopharm. 2016;108:253–61.
Monge-Fuentes V, Muehlmann LA, Longo JPF, Silva JR, Fascineli ML, Azevedo RB, et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J Photochem Photobiol B Biol. 2017;166:301–10.
Pepe D, Carvalho VFM, McCall M, De Lemos DP, Lopes LB. Transportan in nanocarriers improves skin localization and antitumor activity of paclitaxel. Int J Nanomedicine. 2016;11:2009–19.
Guo F, Wang J, Ma M, Tan F, Li N. Skin targeted lipid vesicles as novel nano-carrier of ketoconazole: characterization, in vitro and in vivo evaluation. J Mater Sci Mater Med. 2015;26(4):175.
Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011;25(5):1000–8.
Anarjan N, Nehdi IA, Tan CP. Protection of astaxanthin in astaxanthin nanodispersions using additional antioxidants. Molecules. 2013;18(7):7699–710.
Takebayashi J, Tai A, Gohda E, Yamamoto I. Characterization of the radical-scavenging reaction of 2-O-substituted ascorbic acid derivatives, AA-2G, AA-2P, and AA-2S: a kinetic and stoichiometric study. Biol Pharm Bull. 2006;29(4):766–71.
Elmore AR. Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics. Int J Toxicol. 2005;24(2):51–111.
Fočo A, Gašperlin M, Kristl J. Investigation of liposomes as carriers of sodium ascorbyl phosphate for cutaneous photoprotection. Int J Pharm. 2005;291(1–2):21–9.
Inoue Y, Yoshimura S, Tozuka Y, Moribe K, Kumamoto T, Ishikawa T, et al. Application of ascorbic acid 2-glucoside as a solubilizing agent for clarithromycin: Solubilization and nanoparticle formation. Int J Pharm. 2007;331(1):38–45.
Yoksan R, Jirawutthiwongchai J, Arpo K. Encapsulation of ascorbyl palmitate in chitosan nanoparticles by oil-in-water emulsion and ionic gelation processes. Colloids Surf B: Biointerfaces. 2010;76(1):292–7.
Gopinath D, Ravi D, Rao BR, Apte SS, Renuka D, Rambhau D. Ascorbyl palmitate vesicles (Aspasomes): Formation, characterization and applications. Int J Pharm. 2004;271(1–2):95–113.
Du CB, Liu JW, Su W, Ren YH, Wei DZ. The protective effect of ascorbic acid derivative on PC12 cells: involvement of its ROS scavenging ability. Life Sci. 2003;74(6):771–80.
Murakami K, Inagaki J, Saito M, Ikeda Y, Tsuda C, Noda Y, et al. Skin atrophy in cytoplasmic SOD-deficient mice and its complete recovery using a vitamin C derivative. Biochem Biophys Res Commun. 2009;382(2):457–61.
Yokosawa M, Sonoda Y, Sugiyama S, Saito R, Yamashita Y, Nishihara M, et al. Convection-enhanced delivery of a synthetic retinoid Am80, loaded into polymeric micelles, prolongs the survival of rats bearing intracranial glioblastoma xenografts. Tohoku J Exp Med. 2010;221:257–64.
Kuroda JI, Kuratsu JI, Yasunaga M, Koga Y, Saito Y, Matsumura Y. Potent antitumor effect of SN-38-incorporating polymeric micelle, NK012, against malignant glioma. Int J Cancer. 2009;124(11):2505–11.
Gong J, Chen M, Zheng Y, Wang S, Wang Y. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159(3):312–23.
Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56(9):1273–89.
Remsberg CM, Zhao Y, Takemoto JK, Bertram RM, Davies NM, Forrest ML. Pharmacokinetic evaluation of a DSPE-PEG2000 micellar formulation of ridaforolimus in rat. Pharmaceutics. 2013;5(1):81–93.
Park H, Lee J, Jeong S, Im BN, Kim MK, Yang SG, et al. Lipase-Sensitive Transfersomes Based on Photosensitizer/ Polymerizable Lipid Conjugate for Selective Antimicrobial Photodynamic Therapy of Acne. Adv Healthc Mater. 2016;5(24):3139–47.
Boakye CHA, Patel K, Singh M. Doxorubicin liposomes as an investigative model to study the skin permeation of nanocarriers. Int J Pharm. 2015;489(1–2):106–16.
Tuntiyasawasdikul S, Limpongsa E, Jaipakdee N, Sripanidkulchai B. Transdermal permeation of Kaempferia parviflora methoxyflavones from isopropyl myristate-based vehicles. AAPS Pharm Sci Tech. 2014;15(4):947–55.
Kitagawa S, Tanaka Y, Tanaka M, Endo K, Yoshii A. Enhanced skin delivery of quercetin by microemulsion. J Pharm Pharmacol. 2009;61(7):855–60.
Kitagawa S, Inoue K, Teraoka R, Morita S. Enhanced skin delivery of genistein and other two isoflavones by microemulsion and prevention against UV irradiation-induced erythema formation. Chem Pharm Bull (Tokyo). 2010;58(3):398–401.
Kuwahara K, Kitazawa T, Kitagaki H, Tsukamoto T, Kikuchi M. Nadifloxacin, an antiacne quinolone antimicrobial, inhibits the production of proinflammatory cytokines by human peripheral blood mononuclear cells and normal human keratinocytes. J Dermatol Sci. 2005;38(1):47–55.
Inoue Y, Shimura A, Horage M, Maeda R, Murata I, Sugino M, et al. Effects of the properties of creams on skin penetration. Int J Pharm. 2015;5(3):645–54.
Shinde U, Pokharkar S, Modani S. Design and evaluation of microemulsion gel system of nadifloxacin. Indian J Pharm Sci. 2012;74(3):237–47.
Inoue Y, Matsumoto M, Kimura M, Tanaka T, Kanamoto I. Comparison of the properties of brand-name and generic nadifloxacin creams. Medicina (B Aires). 2011;47(11):616–22.
Yang R, Fu Y, Di Li L, Liu JM. Medium effects on fluorescence of ciprofloxacin hydrochloride. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2003;59(12):2723–32.
Moribe K, Maruyama S, Inoue Y, Suzuki T, Fukami T, Tomono K, et al. Ascorbyl dipalmitate/PEG-lipid nanoparticles as a novel carrier for hydrophobic drugs. Int J Pharm. 2010;387(1–2):236–43.
Moribe K, Tanaka E, Maruyama K, Iwatsuru M. Enhanced Encapsulation of Amphotericin B into Liposomes by Complex Formation with Polyethylene Glycol Derivatives. Pharm Res. 1998;15(11):1737–42.
Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–23.
Saberi AH, Fang Y, Mcclements DJ. Effect of Salts on Formation and Stability of Vitamin E - Enriched Mini- emulsions Produced by Spontaneous Emulsification. J Agric Food Chem. 2014;62(46):11246–53.
Márquez AL, Medrano A, Panizzolo LA, Wagner JR. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J Colloid Interface Sci. 2010;341(1):101–8.
Silvander M, Hellström A, Wärnheim T, Claesson P. Rheological properties of phospholipid-stabilized parenteral oil-in-water emulsions - Effects of electrolyte concentration and presence of heparin. Int J Pharm. 2003;252(1–2):123–32.
Sandström MC, Johansson E, Edwards K. Influence of preparation path on the formation of discs and threadlike micelles in DSPE-PEG2000/lipid systems. Biophys Chem. 2008;132(2–3):97–103.
Johnsson M, Hansson P, Edwards K. Spherical micelles and other self-assembled structures in dilute aqueous mixtures of Poly (ethylene glycol) lipid. J Phys Chem B. 2001;105:8420–30.
Tokudome Y, Uchida R, Yokote T, Todo H, Hada N, Kon T, et al. Effect of topically applied sphingomyelin-based liposomes on the ceramide level in a three-dimensional cultured human skin model. J Liposome Res. 2010;20(1):49–54.
Villasmil-Sánchez S, Rabasco AM, González-Rodríguez ML. Thermal and 31P-NMR studies to elucidate sumatriptan succinate entrapment behavior in phosphatidylcholine/cholesterol liposomes. Comparative 31P-NMR analysis on negatively and positively-charged liposomes. Colloids Surf B: Biointerfaces. 2013;105:14–23.
Eichner A, Stahlberg S, Sonnenberger S, Lange S, Dobner B, Ostermann A, et al. Influence of the penetration enhancer isopropyl myristate on stratum corneum lipid model membranes revealed by neutron diffraction and 2H NMR experiments. Biochim Biophys Acta Biomembr. 2017;1859(5):745–55.
Leal C, Rögnvaldsson S, Fossheim S, Nilssen EA, Topgaard D. Dynamic and structural aspects of PEGylated liposomes monitored by NMR. J Colloid Interface Sci. 2008;325(2):485–93.
Voronov VK. NMR spectra transformed by electron-nuclear coupling as indicator of structural peculiarities of magnetically active molecular systems. J Phys Chem A. 2016;120(34):6688–92.
Todo H, Kimura E, Yasuno H, Tokudome Y, Hashimoto F, Ikarashi Y, et al. Permeation pathway of macromolecules and nanospheres through skin. Biol Pharm Bull. 2010;33(8):1394–9.
Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):1–5.
Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Vol. 42. Prog Lipid Res. 2003;42(1):1–36.
Honeywell-Nguyen PL, Bouwstra JA. Vesicles as a tool for transdermal and dermal delivery. Drug Discov Today Technol. 2005;2(1):67–74.
Iino H, Fujii M, Fujino M, Kohara S, Hashizaki K, Kira H, et al. Influence of Characteristics of Oily Vehicle on Skin Penetration of Ufenamate. Biol Pharm Bull. 2017;40(2):220–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, Y., Hibino, M., Murata, I. et al. A Nanocarrier Skin-Targeted Drug Delivery System using an Ascorbic Acid Derivative. Pharm Res 35, 1 (2018). https://doi.org/10.1007/s11095-017-2311-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-017-2311-3