Abstract
Purpose
To systematically analyze shape and size of soluble irreversible aggregates and the effect of aggregate formation on viscosity.
Methods
Online light scattering, refractive index and viscosity detectors attached to HPLC (Viscotek®) were used to study aggregation, molecular weight and intrinsic viscosity of bovine serum albumin (BSA). Irreversible aggregates were generated by heat stress. Bulk viscosity was measured by an oscillating piston viscometer.
Results
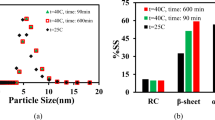
As BSA was heated at a higher concentration or for a longer time, the relative contribution, molecular weight and intrinsic viscosity of aggregate species increased. Molecular shape was evaluated from intrinsic viscosity values, and aggregates were estimated to be more asymmetric than monomer species. The presence of aggregates resulted in an increase in bulk viscosity when relative contribution of very high molecular weight species exceeded 10%.
Conclusions
For model system and conditions studied, generation of higher order aggregate species was concluded to be associated with an increase in molecular asymmetry. Elevated viscosity in the presence of aggregated species points to molecular asymmetry being a critical parameter affecting solution viscosity of BSA.










Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- DP:
-
Differential pressure
- HMW:
-
High molecular weight
- LALS:
-
Low angle light scattering
- mAb:
-
Monoclonal antibody
- mM:
-
Millimolar
- PPI:
-
Protein-protein interactions
- RALS:
-
Right angle light scattering
- RI:
-
Refractive index
- SEC:
-
Size exclusion chromatography
- TDA:
-
Triple detector array
- vHMW:
-
Very high molecular weight
References
Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93:1390–402.
Braun A, Kwee L, Labow MA, Alsenz J. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-α) in normal and transgenic mice. Pharm Res. 1997;14:1472–8.
Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8:E501–7.
Roberts CJ. Protein aggregation and its impact on product quality. Curr Opin Biotechnol. 2014;30:211–7.
Choi S, Park J. Microfluidic rheometer for characterization of protein unfolding and aggregation in microflows. Small. 2010;6:1306–10.
Kovalchuk N, Starov V, Holdich R. Effect of aggregation on viscosity of colloidal suslension. Colloid J. 2010;72:647–52.
Pathak JA, Sologuren RR, Narwal R. Do clustering monoclonal antibody solutions really have a concentration dependence of viscosity? Biophys J. 2013;104:913–23.
Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005;94:1928–40.
Yadav S, Laue TM, Kalonia DS, Singh SN, Shire SJ. The influence of charge distribution on self-association and viscosity behavior of monoclonal antibody solutions. Mol Pharm. 2012;9:791–802.
Ross PD, Minton AP. Hard quasispherical model for the viscosity of hemoglobin solutions. Biochem Biophys Res Commun. 1977;76:971–6.
Mooney M. The viscosity of a concentrated suspension of spherical particles. J Colloid Sci. 1951;6:162–70.
Frensdorff H, Watson M, Kauzmann W. The kinetics of protein denaturation. IV. The viscosity and gelation of urea solutions of Ovalbumin1. J. Am. Chem Soc. 1953;75:5157–66.
Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20:1325–36.
Roberts CJ. Non-native protein aggregation kinetics. Biotechnol Bioeng. 2007;98:927–38.
Sharma VK, Kalonia DS. Experimental detection and characterization of protein aggregates. Aggregation of Ther Proteins. 2010:205–56.
Demeule B, Messick S, Shire SJ, Liu J. Characterization of particles in protein solutions: reaching the limits of current technologies. AAPS J. 2010;12:708–15.
Kalonia C, Kumru OS, Kim JH, Middaugh CR, Volkin DB. Radar chart array analysis to visualize effects of formulation variables on IgG1 particle formation as measured by multiple analytical techniques. J Pharm Sci. 2013;102:4256–67.
Telikepalli SN, Kumru OS, Kalonia C, Esfandiary R, Joshi SB, Middaugh CR, et al. Structural characterization of IgG1 mAb aggregates and particles generated under various stress conditions. J Pharm Sci. 2014;103:796–809.
Kalonia C, Kumru OS, Prajapati I, Mathaes R, Engert J, Zhou S, et al. Calculating the mass of subvisible protein particles with improved accuracy using microflow imaging data. J Pharm Sci. 2015;104:536–47.
Harding SE. The intrinsic viscosity of biological macromolecules. Progress in measurement, interpretation and application to structure in dilute solution. Prog Biophys Mol Biol. 1997;68:207–62.
Harding SE. On the hydrodynamic analysis of macromolecular conformation. Biophys Chem. 1995;55:69–93.
Bajaj H, Sharma VK, Kalonia DS. Determination of second virial coefficient of proteins using a dual-detector cell for simultaneous measurement of scattered light intensity and concentration in SEC-HPLC. Biophys J. 2004;87:4048–55.
Solomon O, Ciutǎ I. Détermination de la viscosité intrinsèque de solutions de polymères par une simple détermination de la viscosité. J Appl Polym Sci. 1962;6:683–6.
Solomon VO, Gotesman B. Zur berechnung der viskositätszahl aus einpunktmessungen. Die Makromol Chem. 1967;104:177–84.
Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci. 2010;99:4812–29.
Joubert MK, Luo Q, Nashed-Samuel Y, Wypych J, Narhi LO. Classification and characterization of therapeutic antibody aggregates. J Biol Chem. 2011;286:25118–33.
Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm. 2005;289:1–30.
Buzzell JG, Tanford C. The effect of charge and ionic strength on the viscosity of ribonuclease. J Phys Chem. 1956;60:1204–7.
Tanford C, Buzzell J. G. The viscosity of aqueous solutions of bovine serum albumin between pH 4.3 and 10.5. J Phys Chem. 1956;60:225–31.
El Kadi N, Taulier N, Le Huerou J, Gindre M, Urbach W, Nwigwe I, et al. Unfolding and refolding of bovine serum albumin at acid pH: ultrasound and structural studies. Biophys J. 2006;91:3397–404.
Curvale R, Masuelli M, Padilla AP. Intrinsic viscosity of bovine serum albumin conformers. Int J Biol Macromol. 2008;42:133–7.
Sarangapani PS, Hudson SD, Migler KB, Pathak JA. The limitations of an exclusively colloidal view of protein solution hydrodynamics and rheology. Biophys J. 2013;105:2418–26.
Squire PG, Moser P, O'Konski CT. Hydrodynamic properties of bovine serum albumin monomer and dimer. Biochem (N Y). 1968;7:4261–72.
Ferrer ML, Duchowicz R, Carrasco B, de la Torre JG, Acuña AU. The conformation of serum albumin in solution: a combined phosphorescence depolarization-hydrodynamic modeling study. Biophys J. 2001;80:2422–30.
Mehl J, Oncley J, Simha R. Viscosity and the shape of protein molecules. Science. 1940;92:132–3.
ACKNOWLEDGMENTS AND DISCLOSURES
Authors would like to thank Lauren Fontana and Rui Fang for help with some experimental aspects, and Elizabeth Zecca for critical reading of the manuscript. Generous material and financial support from Genentech, Inc., as well as Outstanding Scholar and summer fellowships from graduate school at University of Connecticut are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 81 kb)
Rights and permissions
About this article
Cite this article
Pindrus, M.A., Cole, J.L., Kaur, J. et al. Effect of Aggregation on the Hydrodynamic Properties of Bovine Serum Albumin. Pharm Res 34, 2250–2259 (2017). https://doi.org/10.1007/s11095-017-2231-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2231-2