ABSTRACT
Purpose
LY3015014 is a humanized immunoglobulin G4 (IgG4) monoclonal antibody that binds to the catalytic domain of PCSK9 and reduce low-density lipoprotein cholesterol (LDL-C) in patients with hypercholesterolemia that is poorly controlled by maximally tolerated statin therapy. The objective of this pharmacokinetic/pharmacodynamics (PK/PD) analysis was to characterize the PK and PD properties of LY3015014 and assess the effect of covariates on the LY3015014 PK-PD profiles.
Methods
Single and multiple dose data from three phase1 studies in healthy subjects (n = 133), as well as a phase 2 study in hypercholesterolemia patients (n = 527) were combined into a single dataset for analysis. In this dataset, healthy subjects received single intravenous (IV) doses of 0.03 to 10 mg/kg, or multiple subcutaneous (SC) doses of 1.0 to 3.0 mg/kg, administered every 2 to 4 weeks, while patients received 20 to 300 mg every 4 weeks or 100 to 300 every 8 weeks. PK/PD analysis was performed using NONMEM (ICON, software version 7.0 level 3). PK and PD modeling were conducted sequentially, with PK parameters fixed during the development of the PK/PD model. PD parameters and estimated intersubject and intrasubject variability were obtained based on pharmacological drug exposure-response relationships. Age, baseline total PCSK9, body weight, diabetes diagnosis, hypercholesterolemia disease status, dose, ezetimibe administration, gender, ethnic origin, metabolic syndrome, and satin administration were evaluated as potential covariates in the PK model. Baseline total PCSK9, baseline LDL-C, diabetes diagnosis, disease status, ezetimibe administration, gender, ethnic origin, metabolic syndrome, and Statin administration were evaluated as potential covariates in the PD model.
Results
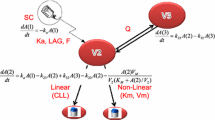
LY3015014 PK profile was consistent across all the studies and between healthy subjects and hypercholesterolemia patients. The PK time course data were well described by a two compartment PK model with first order absorption, and covariates identified for PK parameters included weight on both clearance (CL) and central volume (V2), dose on CL, race on bioavailability (F), and age on V2. The PD (LDL-C) was described using an indirect response model with LY3015014 acting to stimulate the elimination of LDL-C. Covariates identified to have a statistically significant impact on PD were coadministration of statins, baseline LDL-C, metabolic syndrome status and gender.
Conclusions
The population PK/PD model adequately describes the PK and PD profiles of LY3015014. Identification of clinically significant covariates will support the design and dose selection for the pivotal registration studies, ensuring that patients are dosed appropriately.




Similar content being viewed by others
Abbreviations
- CHD:
-
Coronary heart disease
- CL:
-
Clearance
- CV:
-
Cardiovascular
- F:
-
Bioavailability
- HeFH:
-
Heterozygous familial hypercholesterolemia
- IgG4:
-
Immunoglobulin G4
- IV:
-
Intravenous (IV)
- Ka:
-
Rate of absorption
- LDL-C:
-
Low-density lipoprotein cholesterol
- PCSK9:
-
Protein Convertase Subtilisin/kexin Type 9
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- PK/PD:
-
Pharmacokinetic/pharmacodynamics
- Q:
-
Inter-compartmental clearance
- SC:
-
Subcutaneous (SC)
- TMDD:
-
Target mediated drug disposition
- V2:
-
Central volume
- V3:
-
Peripheral volume
REFERENCES
Mackay J, Mensah G, editors. The atlas of heart disease and stroke. Switzerland: WHO; 2004.
Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50(Suppl):S172–7.
Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, et al. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116(11):2995–3005.
Alborn WE, Cao G, Careskey HE, Qian YW, Subramaniam DR, Davies J, et al. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin Chem. 2007;53(10):1814–9.
Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49(2):394–8.
Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–17.
Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation. 2014;129(2):234–43.
Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, et al. Efficacy and safety of the PCSK9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015. doi:10.1016/j.ahj.2015.03.004. Accepted Manuscript.
McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59(25):2344–53.
Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380(9836):29–36.
Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367(20):1891–900.
Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55–61.
Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM, for the ODYSSEY COMBO II Investigators. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated daily statin: results from the ODYSSEY COMBO II study. Eur Heart J. 2015.
Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–40.
Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126(20):2408–17.
Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331–40.
Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380(9858):1995–2006.
Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870–82.
Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015. doi:10.1056/NEJMoa501031.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors would like to acknowledge Eli Lilly’s protocol execution team for their assistance in this study and Leijun Hu, Brian A Willis, Jeffrey S Riesmeyer and Mark A Kryah for their advices.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
(DOCX 73 kb)
Supplemental Figure 2
(DOCX 410 kb)
Supplemental Figure 3
(DOCX 84 kb)
Supplemental Figure 4
(DOCX 400 kb)
Rights and permissions
About this article
Cite this article
Shen, T., James, D.E. & Krueger, K.A. Population Pharmacokinetics (PK) and Pharmacodynamics (PD) Analysis of LY3015014, a Monoclonal Antibody to Protein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Healthy Subjects and Hypercholesterolemia Patients. Pharm Res 34, 185–192 (2017). https://doi.org/10.1007/s11095-016-2054-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2054-6