Abstract
Purpose
Ruthenium complex is a potentially theranostic agent for cancer imaging and therapy, however its application is limited due to poor water-solubility and lack of tumor selectivity. To overcome the above drawbacks, pH-sensitive nanocapsule as a novel targeting carrier was designed to deliver ruthenium complex for treating xenograft tumor of mice.
Methods
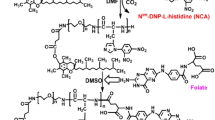
The core/shell structured nanocapsule with ruthenium complex tris(1,10-phenanthroline) ruthenium(II) complex (3P-Ru) as the core and a pH-sensitive polymeric material poly (2-diisopropylaminoethyl methacrylate)-block poly(2-aminoethyl methacrylate hydrochloride) (PbPS) as the shell was synthesized and characterized. Meanwhile, the nanocapsule was used to investigate cell viability and evaluate tissue distribution as well as preventing tumor growth efficacy in U251 stem cells tumor-bearing mouse model.
Results
The nanocapsule had a size of 103.1 ± 11.3 nm, zeta potential of -40 ± 5.3 mV, EE of 76.7 ± 0.9%, LE of 25.4 ± 0.6% and it could control drug release under different pH conditions. The results of cell uptake showed that the fluorescent 3P-Ru loaded in the nanocapsule could be delivered into cells with high efficiency, and then significantly inhibited U251 proliferation in a concentration-dependent manner. After U251 stem cells were transplanted subcutaneously into mice, the 3P-Ru/PbPS nanocapsule (PbPS-Ru-NC) via intravenous administration could concentrate in tumor area and obviously prevent tumor growth.
Conclusions
The pH-sensitive nanocapsule as a antitumor agent carrier was able to effectively deliver 3P-Ru into gliomas cells, and cell growth was significantly inhibited both in vitro and in vivo. Such pH-sensitive nanocapsule for ruthenium complex delivery would have great potential application in tumor theranostics.




Similar content being viewed by others
Abbreviations
- 3P-Ru:
-
Tris(1,10-phenanthroline) ruthenium(II) complex
- AFM:
-
Atomic force microscope
- bFGF:
-
Basic fibroblast growth factor
- DMEM-H:
-
Dulbecco’s Modified Eagle’s Medium (high glucose)
- DMSO:
-
Dimethyl sulfoxide
- EE:
-
Entrapment efficiency
- EGF:
-
Epidermal growth factor
- EPR:
-
Permeability and retention effect
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- LE:
-
Loading efficiency
- MTT:
-
3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide
- MWCO:
-
Molecular weight cut-off
- NC:
-
Nanocapsule
- PAMA/SA:
-
Succinic anhydride modified poly(2-aminoethyl methacrylate hydrochloride)
- PbPS:
-
Poly (2-diisopropylaminoethyl methacrylate) -block poly(2-aminoethyl methacrylate hydrochloride)
- PBS:
-
Phosphate-buffered saline
- PDPA:
-
Poly(2-diisopropylaminoethyl methacrylate)
- PEG:
-
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG2000-NHS)
- SD:
-
Standard deviation
- U251:
-
Human glioma cells
References
Waldt E, Ahlrichs R, Kappes MM, Schooss D. Structures of medium-sized ruthenium clusters: the octahedral motif. Chem Phys Chem. 2014;15(5):862–5.
Cardoso CR, De AI, Camilo MR, Lima MV, Ito AS, Baptista MS, et al. Synthesis, spectroscopic characterization, photochemical and photophysical properties and biological activities of ruthenium complexes with mono- and bi-dentate histamine ligand. Dalton Trans. 2012;41(22):6726–34.
Kostova I. Ruthenium complexes as anticancer agents. Curr Med Chem. 2006;13(9):1085–107.
Brabec V, Novakova Olga. DNA binding mode of ruthenium complexes and relationship to tumor cell toxicity. Drug resist. Update. 2006;9(3):111–122.
Zhang R, Yu X, Ye Z, Wang G, Zhang W, Yuan J. Turn-on luminescent probe for cysteine/homocysteine based on a ruthenium(II) complex. Inorg Chem. 2010;49(17):7898–903.
Gill MR, Thomas JA. Ruthenium(II) polypyridyl complexes and DNA-from structural probes to cellular imaging and therapeutics. Chem Soc Rev. 2012;41(8):3179–92.
Coggan DZ, Haworth IS, Bates PJ, Robinson A, Rodger A. DNA binding of ruthenium tris(1,10-phenanthroline): evidence for the dependence of binding mode on metal complex concentration. Inorg Chem. 1999;38(20):4486–97.
Cardoso CR, Lima MV, Cheleski J, Peterson EJ, Venâncio T, Farrell NP, et al. Luminescent ruthenium complexes for theranostic applications. J Med Chem. 2014;57(11):4906–15.
Süss-Fink G. Water-soluble arene ruthenium complexes: from serendipity to catalysis and drug design. J Organomet Chem. 2014;751(2):2–19.
Stubbs M, McSheehy PMJ, Griffiths JR, Bashford CL. Causes and consequences of tumor acidity and implications for treatment. Mol Med Today. 2000;6(1):15–9.
Prescott DM, Charles HC, Poulson JM, Page RL, Thrall DE, Vujaskovic Z, et al. The relationship between intracellular and extracellular pH in spontaneous canine tumors. Clin Cancer Res. 2000;6(6):2501–5.
Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74(1):47–61.
Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71(3):409–19.
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–10.
Van SR, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdán S, et al. In vivo imaging of extracellular pH using 1H MRSI. Magn Reson Med. 1999;41(4):743–50.
Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5(10):786–95.
Pourjavadi A, Tehrani ZM, Moghanaki AA. Folate-conjugated pH-responsive nanocarrier designed for active tumor targeting and controlled release of Gemcitabine. Pharm Res. 2016;33(2):417–32.
Tran TH, Ramasamy T, Choi JY. Tumor-targeting, pH-sensitive nanoparticles for docetaxel delivery to drug-resistant cancer cells. Int J Nanomedecine. 2015;10(1):5249–62.
Zhou Z, Li L, Yang Y, Xu X, Huang Y. Tumor targeting by pH-sensitive, biodegradable, cross-linked N-(2-hydroxypropyl) methacrylamide copolymer micelles. Biomaterials. 2014;35(24):6622–35.
Wu W, Zhang Q, Wang J, Chen M, Li S, Lin Z, et al. Tumor-targeted aggregation of pH-sensitive nanocarriers for enhanced retention and rapid intracellular drug release. Polym Chem. 2014;5(19):5668–79.
Wang Q, Wang Z, Chu L, Li X, Kan P, Xin X, et al. The effects and molecular mechanisms of MiR-106a in multidrug resistance reversal in human glioma U87/DDP and U251/G cell lines. PLoS One. 2015;10(5), e0125473.
Li T, Fu C, Liu Z, Guo S, Liu Z, Wen TB. Half-Sandwich B-Oxy Boratabenzene ruthenium complexes: synthesis, characterization, and reactivity of (η6-C5H5BOR)RuCl(PPh3)2 (R = Et, Me). Organometallics. 2015;34(13):3292–302.
Gao Y, Chao Z, Zhou Y, Li J, Lei Z, Li Y, et al. Endosomal pH-responsive polymer-based dual-ligand-modified micellar nanoparticles for tumor targeted delivery and facilitated intracellular release of paclitaxel. Pharm Res. 2015;32(8):2649–62.
Kastantin M, Missirlis D, Black M, Ananthanarayanan B, Peters D, Tirrell M. Thermodynamic and kinetic stability of DSPE-PEG(2000) micelles in the presence of bovine serum albumin. J Phys Chem B. 2010;114(39):12632–40.
Cesur H, Rubinstein I, Pai A, Önyüksel H. Self-associated indisulam in phospholipid-based nanomicelles: a potential nanomedicine for cancer. Nanomed-Nanotechnol. 2009;5(2):178–83.
Hua S. Comparison of in vitro dialysis release methods of loperamide-encapsulated liposomal gel for topical drug delivery. Int J Nanomedicine. 2014;9(1):735–44.
Xu P, Qiu M, Zhang Z, Kang C, Jiang R, Jia Z, et al. The oncogenic roles of Notch1 in astrocytic gliomas in vitro and in vivo. J Neuro-Oncol. 2010;97(1):41–51.
Bouskila A, Amouyal E, Verchère-Béaur C, Sasaki I, Gaudemer A. Mononuclear and binuclear ruthenium(II) heteroleptic complexes based on 1,10-phenanthroline ligands. Part II: spectroscopic and photophysical study in the presence of DNA. J Photochem Photobiol B. 2004;76(1-3):69–83.
Ruzzaa A, Vespignania R, Khoynezhada A, Bercic G, De Robertisa M, Trentoa A, et al. Concomitant dual intravascular and subcutaneous microsurgical implantation of xenograft tissue in a rodent model for evaluation of structural degeneration. Transplant Proc. 2013;45(2):735–40.
Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–46.
Linton SS, Sherwood SG, Drews KC, Kester M. Targeting cancer cells in the tumor microenvironment: opportunities and challenges in combinatorial nanomedicine. Wires Nanomed Nanobiol. 2016;8(2):208–22.
ACKNOWLEDGMENTS & DISCLOSURES
This work was supported by grants from the Natural Science Foundation of China (81273416); the Scientific Research Foundation for Returned Scholars, Ministry of Education of China (2012-940), the Fundamental Research Funds for the Central Universities (XDJK2013A030); and the New Century Excellent Talents in University Award, Ministry of Education of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Fu, C., Deng, Y. et al. A pH-Sensitive Nanocarrier for Tumor Targeting. Pharm Res 33, 2989–2998 (2016). https://doi.org/10.1007/s11095-016-2021-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2021-2