Abstract
Purpose
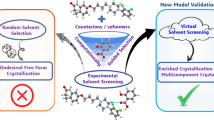
Solvates are mainly undesired by-products during the pharmaceutical development of new drugs. In addition, solvate formation may also distort solubility measurements. The presented study introduces a simple computational approach that allows for the identification of drug solvent pairs which most likely form crystalline solid phases.
Methods
The mixing enthalpy as a measure for drug-solvent complementarity is obtained by computational liquid phase thermodynamics (COSMO-RS theory). In addition a few other simple descriptors were taking into account describing the shape and topology of the drug and the solvent. Using an extensive dataset of drug solvent pairs a simple and statistically robust model is developed which allows for a rough assessment of a solvent’s ability to form a solvate.
Results
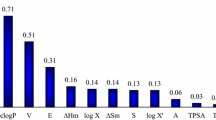
Similar to the related issue of cocrystal screening, the mixing (or excess) enthalpy of the subcooled liquid mixture of the drug-solvent pair proves to be an important quantity controlling solvate formation. Due to the fact that many solvates form inclusion compounds, the solvent shape is another important factor influencing solvate formation. Solvates forming channel-like voids in the solid state are predicted less well.
Conclusion
The approach ranks any drug-solvent pair that forms a solvate before any non-solvate by a probability of about 81% (AUC = 0.81), giving a significant advantage over any trial and error approach. Hence it can help to identify suitable solvent candidates early in the drug development process.




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the ROC curve
- COSMO:
-
Conductor like screening model
- COSMO-RS:
-
COSMO for realistic solvation
- G ex :
-
Excess free energy
- H kmix :
-
Enthalpy of compound k in mixture
- H kpure :
-
Enthalpy of pure compound k
- H ex :
-
Excess enthalpy
- log10(x):
-
logarithm of mole fraction solubility
- n ring , drug :
-
Number of ring atoms of drug
- O solvent :
-
Ovality index of solvent
- p solvate :
-
Probability of solvate formation
- ROC:
-
Receiver operating characteristic
- V COSMO :
-
Volume of molecular COSMO cavity
- x :
-
Mole fraction
- ΔG fus :
-
Gibbs free energy of fusion
- ΔG mix :
-
Gibbs free energy of mixing
- ΔG solvate :
-
Gibbs free energy of solvate formation
- ΔH fus :
-
Enthalpy of fusion
- ΔH mix :
-
Mixing enthalpy
References
Hilfiker R, editor. Polymorphism in the pharmaceutical industry. Weinheim: Wiley-VCH; 2006.
Korobov MV, Mirakyan AL, Avramenko NV, Olofsson G, Smith AL, Ruoff RS. Calorimetric studies of solvates of C 60 and C 70 with aromatic solvents. J Phys Chem B. 1999;103:1339–46.
Threlfall TL. Analysis of organic polymorphs. A review. Analyst. 1995;120:2435–60.
McCrone WC. Physics and chemistry of the organic solid state. Physics and chemistry of the organic solid state. London: Interscience Publishers; 1965. p. 725–67.
Nangia A, Desiraju GR. Pseudopolymorphism: occurrences of hydrogen bonding organic solvents in molecular crystals. Chem Commun. 1999;605–6.
Takieddin K, Khimyak YZ, Fábián L. Prediction of hydrate and solvate formation using statistical models. Cryst Growth Des. 2016;16:70–81.
Johnston A, Johnston BF, Kennedy AR, Florence AJ. Targeted crystallisation of novel carbamazepine solvates based on a retrospective Random Forest classification. CrystEngComm. 2008;10:23.
Abramov YA, Loschen C, Klamt A. Rational coformer or solvent selection for pharmaceutical cocrystallization or desolvation. J Pharm Sci. 2012;101:3687.
Loschen C, Klamt A. Solubility prediction, solvate and cocrystal screening as tools for rational crystal engineering: solubility prediction, solvate and cocrystal screening. J Pharm Pharmacol. 2015;67:803–11.
Abramov YA. Virtual hydrate screening and coformer selection for improved relative humidity stability. CrystEngComm. 2015;17:5216–24.
am Ende D, Klamt A, Loschen C, Anderson S, Salan JS, Dube P, et al. Prediction of energetic-energetic cocrystal forms and their competition with solvate formation [Internet]. Atlanta, GA, USA; 2014. Available from: http://www.aiche.org/conferences/aiche-annual-meeting/2014.
Klamt A. Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem. 1995;99:2224–35.
Klamt A, Krooshof GJP, Taylor R. COSMOSPACE: alternative to conventional activity-coefficient models. AIChE J. 2002;48:2332–49.
Klamt A. The COSMO and COSMO-RS solvation models. WIREs Comput Mol Sci. 2011;1:699–709.
U.S. Department of Health and Human Services, Guidance for Industry, Q3C - Tables and List, 2012. [Internet]. U.S. Department of Health and Human Services, Guidance for Industry, Q3C - Tables and List. 2014 [cited 2014 May 19]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComp lianceRegulatoryInformation/ Guidances/ucm073395.pdf.
Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning: data mining, inference, and prediction. Springer; 2001.
Herschtal A, Raskutti B. Optimising area under the ROC curve using gradient descent. Proceedings of the Twenty-first International Conference on Machine Learning [Internet]. New York, NY, USA: ACM; 2004. p. 49. Available from: http://doi.acm.org/10.1145/1015330.1015366.
COSMOtherm C3.0, release 15.01. Leverkusen, Germany: COSMOlogic GmbH & Co. KG; 2014. http://www.cosmologic.de.
TURBOMOLE 7.0. Karlsruhe, Germany: University of Karlsruhe and Forschungszentrum Karlsruhe GmbH (1989–2007), TURBOMOLE GmbH (since 2007); 2015. http://www.turbomole.com.
Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A. 1988;38:3098–100.
Perdew JP. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B. 1986;33:8822–4.
Klamt A, Eckert F, Diedenhofen M. Prediction of the free energy of hydration of a challenging set of pesticide-like compounds†. J Phys Chem B. 2009;113:4508–10.
R Core Team. R: a language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2013. Available from: http://www.R-project.org/.
Be̅rziņš A, Skarbulis E, Rekis T, Actiņš A. On the formation of droperidol solvates: characterization of structure and properties. Cryst Growth Des. 2014;14:2654–64.
Bingham AL, Hughes DS, Hursthouse MB, Lancaster RW, Tavener S, Threlfall TL. Over one hundred solvates of sulfathiazole. Chem Commun. 2001;603–4.
Nauha E, Saxell H, Nissinen M, Kolehmainen E, Schafer A, Schlecker R. Polymorphism and versatile solvate formation of thiophanate-methyl. CrystEngComm. 2009;11:2536–47.
Stieger N, Liebenberg W, Wessels J, Samsodien H, Caira M. Channel inclusion of primary alcohols in isostructural solvates of the antiretroviral nevirapine: an X-ray and thermal analysis study. Struct Chem. 2010;21:771–7.
Wicker JGP, Cooper RI. Will it crystallise? Predicting crystallinity of molecular materials. CrystEngComm. 2015;17:1927–34.
Hosokawa T, Datta S, Sheth AR, Brooks NR, Young VG, Grant DJW. Isostructurality among five solvates of phenylbutazone. Cryst Growth Des. 2004;4:1195–201.
Datta S, Grant DJW. Crystal structures of drugs: advances in determination, prediction and engineering. Nat Rev Drug Discov. 2004;3:42–57.
ACKNOWLEDGMENTS AND DISCLOSURES
We would like to thank Yuriy A. Abramov, Pfizer and David am Ende, Nalas Engineering for fruitful discussions.
The authors declare the following competing financial interest(s): Andreas Klamt is CEO and Christoph Loschen is an employee of COSMOlogic.
COSMOlogic develops and commercially distributes the COSMOtherm software package used in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loschen, C., Klamt, A. Computational Screening of Drug Solvates. Pharm Res 33, 2794–2804 (2016). https://doi.org/10.1007/s11095-016-2005-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2005-2