Abstract
Purpose
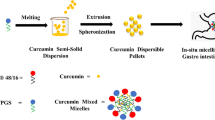
The aim of our study was development of advanced third generation Curcumin self microemulsifying composition solid dispersion (Cur SMEC-SD) with high drug loading, improved stability, rapid in-vitro dissolution and enhanced bioavailability for improved therapy of rheumatoid arthritis.
Method
The Cur SMEC-SD comprising polymers (KollidonVA64[KVA], Eudragits, HPMC and Soluplus) and self microemulsifying composition of surfactant:co-surfactant:oil were coated onto rapidly disintegrating inert tablet core. SDs evaluated for stability, in-vitro release and bioenhancement.
Results
Cur SMEC-SDs exhibited high Cur loading of 45% w/w and microemulsion formation with globule size (~100 nm) irrespective of polymers. Among the polymers, SD with KVA revealed exceptionally low contact angle (7°C) and rapid in-vitro release (t50%-6.45 min). No crystallization was evident as confirmed by SEM, DSC and XRD and is attributed to SMEC aided solubilization/amorphisation, and interaction of KVA with Cur seen in the FTIR spectra. Stability was confirmed as per ICH guidelines. Remarkable bioenhancement with Cur SMEC-SD was confirmed by the > four fold and a two fold compared to Cur and Cur-SD without SMEC respectively. High efficacy ~ 80% compared to Indomethacin, seen with rheumatoid arthritis (RA) induced rats coupled with no adverse toxicity.
Conclusion
The advanced third generation Cur SMEC-SD presents a practical technological advancement and suggests Cur SMEC-SD as promising alternative for RA therapy.















Similar content being viewed by others
Abbreviations
- CFA:
-
Complete Freund’s adjuvant
- Cur:
-
Curcumin
- DSC:
-
Differential scanning calorimetric
- KVA:
-
Kollidon VA 64
- Cmax :
-
Peak drug concentration
- RBC:
-
Red blood cells
- RF:
-
Rheumatoid factor
- SEM:
-
Scanning electron microscopy
- SMEC:
-
Self-microemulsifying composition
- CRP:
-
Serum C reactive protein
- SD:
-
Solid dispersion
- WBC:
-
White blood cells
- XRD:
-
X ray powder diffraction
References
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18.
Seo S-W, Han H-K, Chun M-K, Choi H-K. Preparation and pharmacokinetic evaluation of curcumin solid dispersion using Solutol® HS15 as a carrier. Int J Pharm. 2012;424(1):18–25.
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin–phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330(1):155–63.
Gong C, Deng S, Wu Q, Xiang M, Wei X, Li L, et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials. 2013;34(4):1413–32.
Bergonzi M, Hamdouch R, Mazzacuva F, Isacchi B, Bilia A. Optimization, characterization and in vitro evaluation of curcumin microemulsions. LWT-Food Sci Technol. 2014;59(1):148–55.
Kakkar V, Mishra AK, Chuttani K, Kaur IP. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. Int J Pharm. 2013;448(2):354–9.
Nguyen TN-G, Tran PH-L, Van Tran T, Van Vo T, Truong-DinhTran T. Development of a modified–solid dispersion in an uncommon approach of melting method facilitating properties of a swellable polymer to enhance drug dissolution. Int J Pharm. 2015;484(1):228–34.
Li B, Konecke S, Wegiel LA, Taylor LS, Edgar KJ. Both solubility and chemical stability of curcumin are enhanced by solid dispersion in cellulose derivative matrices. Carbohydr Polym. 2013;98(1):1108–16.
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12(23):1068–75.
Vo CL-N, Park C, Lee B-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3):799–813.
Won D-H, Kim M-S, Lee S, Park J-S, Hwang S-J. Improved physicochemical characteristics of felodipine solid dispersion particles by supercritical anti-solvent precipitation process. Int J Pharm. 2005;301(1):199–208.
Dannenfelser RM, He H, Joshi Y, Bateman S, Serajuddin A. Development of clinical dosage forms for a poorly water soluble drug I: application of polyethylene glycol–polysorbate 80 solid dispersion carrier system. J Pharm Sci. 2004;93(5):1165–75.
Zaki RM, Ali AA, El Menshawi SF, Bary AA. Effect of binary and ternary solid dispersions prepared by fusion method on the dissolution of poorly water soluble diacerein. Int J Drug Delivery. 2013;5(1):99.
Yu D-G, Yang J-M, Branford-White C, Lu P, Zhang L, Zhu L-M. Third generation solid dispersions of ferulic acid in electrospun composite nanofibers. Int J Pharm. 2010;400(1):158–64.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3):278–87.
Heo M-Y, Piao Z-Z, Kim T-W, Cao Q-R, Kim A, Lee B-J. Effect of solubilizing and microemulsifying excipients in polyethylene glycol 6000 solid dispersion on enhanced dissolution and bioavailability of ketoconazole. Arch Pharm Res. 2005;28(5):604–11.
Beg S, Sharma G, Thanki K, Jain S, Katare O, Singh B. Positively charged self-nanoemulsifying oily formulations of olmesartan medoxomil: systematic development, in vitro, ex vivo and in vivo evaluation. Int J Pharm. 2015;493(1):466–82.
Boateng JS, Matthews KH, Auffret AD, Humphrey MJ, Stevens HN, Eccleston GM. In vitro drug release studies of polymeric freeze-dried wafers and solvent-cast films using paracetamol as a model soluble drug. Int J Pharm. 2009;378(1):66–72.
Nascimento A, Neto EB, Moura M, Dantas TC, Neto AD. Wettability of paraffin surfaces by nonionic surfactants: Evaluation of surface roughness and nonylphenol ethoxylation degree. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2014.
Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm. 2010;76(3):475–85.
Chopra S, Venkatesan N, Betageri GV. Formulation of lipid bearing pellets as a delivery system for poorly soluble drugs. Int J Pharm. 2013;446(1):136–44.
Banji D, Pinnapureddy J, Banji OJ, Saidulu A, Hayath MS. Synergistic activity of curcumin with methotrexate in ameliorating Freund’s complete adjuvant induced arthritis with reduced hepatotoxicity in experimental animals. Eur J Pharmacol. 2011;668(1):293–8.
Zhang L, Zhu W, Yang C, Guo H, Yu A, Ji J, et al. A novel folate-modified self-microemulsifying drug delivery system of curcumin for colon targeting. Int J Nanomedicine. 2012;7:151.
Dixit R, Puthli S. Oral strip technology: overview and future potential. J Control Release. 2009;139(2):94–107.
Hu X, Lin C, Chen D, Zhang J, Liu Z, Wu W, et al. Sirolimus solid self-microemulsifying pellets: formulation development, characterization and bioavailability evaluation. Int J Pharm. 2012;438(1):123–33.
Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31(25):6597–611.
Zeng Z, Sun L, Xue W, Yin N, Zhu W. Relationship of intrinsic viscosity to molecular weight for poly (1, 4-butylene adipate). Polym Test. 2010;29(1):66–71.
Moon D-O, Kim M-O, Choi YH, Park Y-M, Kim G-Y. Curcumin attenuates inflammatory response in IL-1β-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol. 2010;10(5):605–10.
Obiri DD, Osafo N, Ayande PG, Antwi AO. Xylopia aethiopica (Annonaceae) fruit extract suppresses Freund’s adjuvant-induced arthritis in Sprague–Dawley rats. J Ethnopharmacol. 2014;152(3):522–31.
Ward JR, Cloud RS. Comparative effect of antirheumatic drugs on adjuvant-induced polyarthritis in rats. J Pharmacol Exp Ther. 1966;152(1):116–21.
Bani S, Kaul A, Khan B, Gupta VK, Satti NK, Suri KA, et al. Anti-arthritic activity of a biopolymeric fraction from Euphorbia tirucalli. J Ethnopharmacol. 2007;110(1):92–8.
Behar SM, Porcelli SA. Mechanisms of autoimmune disease induction. Arthritis Rheumatism. 1995;38(4):458–76.
Kumar DA, Manikandan P, Sumitra M, Raju KVN, Gayathri C, Arutselvan N, et al. A novel peptide derivative exhibits antiinflammatory and antioxidant activity in adjuvant induced arthritis in rats. Mol Cell Biochem. 2002;229(1–2):9–17.
Ridker P. Should statin therapy be considered for patients with elevated C-reactive protein? The need for a definitive clinical trial. Eur Heart J. 2001;23(22):2135–7.
Bauerova K, Acquaviva A, Ponist S, Gardi C, Vecchio D, Drafi F, et al. Markers of inflammation and oxidative stress studied in adjuvant-induced arthritis in the rat on systemic and local level affected by pinosylvin and methotrexate and their combination. Autoimmunity. 2015;48(1):46–56.
ACKNOWLEDGMENTS AND DISCLOSURES
Authors are thankful to Phoenix Pharmaceuticals LLC, USA for providing fellowship to Prashant Mande.
Phoenix Pharmaceuticals LLC, USA
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mande, P.P., Bachhav, S.S. & Devarajan, P.V. Solid Dispersion of Curcumin as Polymeric Films for Bioenhancement and Improved Therapy of Rheumatoid Arthritis. Pharm Res 33, 1972–1987 (2016). https://doi.org/10.1007/s11095-016-1934-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1934-0