Abstract
Purpose
This study tested the hypothesis that encapsulation of influenza vaccine in microneedle patches increases vaccine stability during storage at elevated temperature.
Methods
Whole inactivated influenza virus vaccine (A/Puerto Rico/8/34) was formulated into dissolving microneedle patches and vaccine stability was evaluated by in vitro and in vivo assays of antigenicity and immunogenicity after storage for up to 3 months at 4, 25, 37 and 45°C.
Results
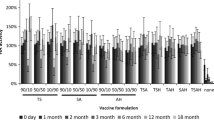
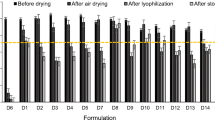
While liquid vaccine completely lost potency as determined by hemagglutination (HA) activity within 1–2 weeks outside of refrigeration, vaccine in microneedle patches lost 40–50% HA activity during or shortly after fabrication, but then had no significant additional loss of activity over 3 months of storage, independent of temperature. This level of stability required reduced humidity by packaging with desiccant, but was not affected by presence of oxygen. This finding was consistent with additional stability assays, including antigenicity of the vaccine measured by ELISA, virus particle morphological structure captured by transmission electron microscopy and protective immune responses by immunization of mice in vivo.
Conclusions
These data show that inactivated influenza vaccine encapsulated in dissolving microneedle patches has enhanced stability during extended storage at elevated temperatures.










Similar content being viewed by others
Abbreviations
- HA:
-
Hemagglutination
- HRP:
-
Horseradish peroxidase
- IACUC:
-
Institutional animal care and use committee
- OD:
-
Optical density
- PBS:
-
Phosphate-buffered saline
- PBST:
-
Phosphate-buffered saline plus 0.5% Tween-20
- PDMS:
-
Polydimethylsiloxane
- PVA:
-
Polyvinyl alcohol
- RBC:
-
Red blood cell
- s.d.:
-
Standard deviation
- TMB:
-
Tetramethylbenzidine
- TEM:
-
Transmission electron microscopy
References
Plotkin SA, Orenstein W, Offit PA, editors. Vaccines. Philadelphia, PA: Saunders; 2012.
Chen D, Kristensen D. Opportunities and challenges of developing thermostable vaccines. Expert Rev Vaccines. 2009;8(5):547–57.
Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine. 2007;25(20):3980–6.
Services USDoHaH. HHS pandemic influenza plan. Washington, DC: U.S. Department of Health and Human Services; 2005.
Amorij JP, Huckriede A, Wilschut J, Frijlink HW, Hinrichs WL. Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharm Res. 2008;25(6):1256–73.
Remmele RL, Krishnan S, Callahan WJ. Development of stable lyophilized protein drug products. Curr Pharm Biotechnol. 2012;13(3):471–96.
Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46(1–3):307–26.
Chang LL, Pikal MJ. Mechanisms of protein stabilization in the solid state. J Pharm Sci. 2009;98(9):2886–908.
Geeraedts F, Saluja V, ter Veer W, Amorij JP, Frijlink HW, Wilschut J, et al. Preservation of the immunogenicity of dry-powder influenza H5N1 whole inactivated virus vaccine at elevated storage temperatures. AAPS J. 2010;12(2):215–22.
Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of blood-borne pathogens. Bull World Health Organ. 1999;77(10):789–800.
Donnelly R, Douroumis D. Microneedles for drug and vaccine delivery and patient monitoring. Drug Deliv Transl Res. 2015;5(4):311–2.
Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–68.
Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol. 2013;785:121–32.
Birchall JC, Clemo R, Anstey A, John DN. Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28(1):95–106.
Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–62.
Kommareddy S, Baudner BC, Oh S, Kwon SY, Singh M, O’Hagan DT. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharm Sci. 2012;101(3):1021–7.
Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24.
Sullivan SP, Koutsonanos DG, Del Pilar MM, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–20.
Koutsonanos DG, del Pilar MM, Zarnitsyn VG, Jacob J, Prausnitz MR, Compans RW, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 2011;204(4):582–91.
Pearton M, Pirri D, Kang SM, Compans RW, Birchall JC. Host responses in human skin after conventional intradermal injection or microneedle administration of virus-like-particle influenza vaccine. Adv Healthc Mater. 2013;2(10):1401–10.
Pulit-Penaloza JA, Esser ES, Vassilieva EV, Lee JW, Taherbhai MT, Pollack BP, et al. A protective role of murine langerin(+) cells in immune responses to cutaneous vaccination with microneedle patches. Sci Rep. 2014;4:6094.
Song JM, Kim YC, Lipatov AS, Pearton M, Davis CT, Yoo DG, et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol. 2010;17(9):1381–9.
Zaric M, Lyubomska O, Poux C, Hanna ML, McCrudden MT, Malissen B, et al. Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and Th1 immune responses by murine Langerhans cells. J Investig Dermatol. 2015;135(2):425–34.
DeMerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem Toxicol. 2003;41(3):319–26.
Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74(11):4999–5005.
Chu LY, Choi SO, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubbles and pedestal designs. J Pharm Sci. 2010;99(10):4228–38.
Wen Z, Ye L, Gao Y, Pan L, Dong K, Bu Z, et al. Immunization by influenza virus-like particles protects aged mice against lethal influenza virus challenge. Antivir Res. 2009;84(3):215–24.
Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106(19):7968–73.
Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–95.
Cicerone MT, Pikal MJ, Qian KK. Stabilization of proteins in solid form. Adv Drug Deliv Rev. 2015;93:14–24.
Clements CJ, Larsen G, Jodar L. Technologies that make administration of vaccines safer. Vaccine. 2004;22(15–16):2054–8.
Brandau DT, Jones LS, Wiethoff CM, Rexroad J, Middaugh CR. Thermal stability of vaccines. J Pharm Sci. 2003;92(2):218–31.
Bieganski RM, Fowler A, Morgan JR, Toner M. Stabilization of active recombinant retroviruses in an amorphous dry state with trehalose. Biotechnol Prog. 1998;14(4):615–20.
Schebor C, Burin L, Buera MP, Aguilera JM, Chirife J. Glassy state and thermal inactivation of invertase and lactase in dried amorphous matrices. Biotechnol Prog. 1997;13(6):857–63.
Hinrichs WL, Prinsen MG, Frijlink HW. Inulin glasses for the stabilization of therapeutic proteins. Int J Pharm. 2001;215(1–2):163–74.
Chen X, Fernando GJ, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, et al. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Control Release. 2011;152(3):349–55.
Choi HJ, Yoo DG, Bondy BJ, Quan FS, Compans RW, Kang SM, et al. Stability of influenza vaccine coated onto microneedles. Biomaterials. 2012;33(14):3756–69.
Hirobe S, Azukizawa H, Hanafusa T, Matsuo K, Quan YS, Kamiyama F, et al. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials. 2015;57:50–8.
Mistilis MJ, Bommarius AS, Prausnitz MR. Development of a thermostable microneedle patch for influenza vaccination. J Pharm Sci. 2015;104(2):740–9.
Vassilieva EV, Kalluri H, McAllister D, Taherbhai MT, Esser ES, Pewin WP, et al. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Deliv Transl Res. 2015;5(4):360–71.
Soema PC, Willems GJ, van Twillert K, van de Wijdeven G, Boog CJ, Kersten GF, et al. Solid bioneedle-delivered influenza vaccines are highly thermostable and induce both humoral and cellular immune responses. PLoS One. 2014;9(3):e92806.
ACKNOWLEDGMENTS AND DISCLOSURES
We would like to thank Seong-O Choi for providing the pyramidal microneedle master structure and Donna Bondy for administrative support. This work was supported in part by the National Institutes of Health. The work was carried out at the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology, and at the Emory Vaccine Center. Mark Prausnitz is an inventor on patents and has a significant financial interest in a company that is developing microneedle-based products (Micron Biomedical). This potential conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, L.Y., Ye, L., Dong, K. et al. Enhanced Stability of Inactivated Influenza Vaccine Encapsulated in Dissolving Microneedle Patches. Pharm Res 33, 868–878 (2016). https://doi.org/10.1007/s11095-015-1833-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1833-9