ABSTRACT
Purpose
The surface charge of nanoparticles is an important factor that controls efficiency and cellular uptake. The aim of this study was to investigate the efficacy of curcumin nanoparticles (Cur-NPs) with different surface charges, in terms of toxicity, internalization, anti-inflammatory and anti-oxidant activities towards alveolar macrophages cells.
Methods
The surface charge of curcumin nanoparticles (positive, negative and neutral), with an average diameter of 30 nm, were synthesized and characterized. Polyvinyl-alcohol, polyvinylpyrrolidone and dextran were used as coatings to confer negative, positive and neutral charges. The synthesized Cur-NPs were evaluated for particle size, encapsulation efficiency, surface charge, qualitative and quantitative cellular uptakes, anti-oxidant and anti-inflammatory activities.
Results
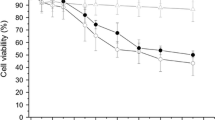
Positively charged nanoparticles showed higher cytotoxicity effects compared to negative and neutral particles. The same trend was observed in antioxidant activity, which included radical scavenging and nitric oxide production. In addition, the anti-inflammatory activity (interleukin-1β, IL-6 and TNF-α) depleted in the order: positive>negative>neutral. The void neutral-, positively- and negatively-charged nanoparticles did not show any cytotoxic effects.
Conclusion
The difference in activity for different surface charges of Cur-NPs may be due to the internalization rate of the particles by alveolar macrophages. Intracellular uptake measurements demonstrated that Cur-NPs with positive surface charges possessed the strongest interaction with alveolar macrophages.









Similar content being viewed by others
Abbreviations
- CLSM:
-
Confocal laser scanning microscope
- CUR:
-
Curcumin
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DMSO:
-
Dimethyl sulfoxide
- DNA:
-
Deoxyribonucleic acid
- DPPH:
-
1,1-diphenyl-2-picryhydrazyl
- FBS:
-
Fetal bovine serum
- HDAC2:
-
Histone deacetylase-2
- IL-17:
-
Interleukin-17
- IL-1β:
-
Interleukin-1beta
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- iNOS:
-
Induciblcbve nitric oxide synthase
- L-NAME:
-
L-nitro-arginine methyl ester
- LPS:
-
Lipopolysaccharide
- MCP-1:
-
Monocyte chemotactic protein-1
- MIP-1α:
-
Monocyte inflammatory protein-1
- MTS:
-
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolim
- Mw :
-
Molecular weight
- NF-κB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- NPs:
-
Nanoparticles
- PBS:
-
Phosphate buffer saline
- PDI:
-
Polydispersity index
- PLGA:
-
Poly(lactic-co-glycolic) acid
- PVA:
-
Polyvinyl alcohol
- PVP:
-
Polyvinylpyrrolidone
- TEM:
-
Transmission electron microscope
- TNF-α:
-
Tumor necrosis factor alpha
- VP:
-
Void particles
References
Biswas S, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.
Lauzon-Joset JF, Marsolais D, Langlois A, Bissonnette EY. Dysregulation of alveolar macrophages unleashes dendritic cell-mediated mechanisms of allergic airway inflammation. Mucosal Immunol. 2014;7(1):155–64.
Furuie H, Yamasaki H, Suga M, Ando M. Altered accessory cell function of alveolar macrophages: a possible mechanism for induction of Th2 secretory profile in idiopathic pulmonary fibrosis. Eur Respir J. 1997;10(4):787–94.
Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107(12):1529–36.
Jayachandran R, BoseDasgupta S, Pieters J. Surviving the macrophage: tools and tricks employed by Mycobacterium tuberculosis. Curr Top Microbiol. 2014;374:189–209.
Lee W-H, Loo C-Y, Bebawy M, Luk F, Mason RS, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol. 2013;11(4):338–78.
Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–99.
Shehzad A, Khan S, Lee YS. Curcumin molecular targets in obesity and obesity-related cancers. Future Oncol. 2012;8(2):179–90.
Lee WH, Bebawy M, Loo CY, Luk F, Mason RS, Rohanizadeh R. Fabrication of curcumin micellar nanoparticles with enhanced anti-cancer activity. J Biomed Nanotechnol. 2015;11(13):1093–105.
Lee W-H, Loo C-Y, Young PM, Traini D, Mason RS, Rohanizadeh R. Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin Drug Deliv. 2014;11(8):1183–201.
Jung KK, Lee HS, Cho JY, Shin WC, Rhee MH, Kim TG, et al. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006;79(21):2022–31.
Zhang LJ, Wu CF, Zhao SQ, Yuan D, Lian GN, Wang XX, et al. Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-kappa B signaling pathways in N9 microglia induced by lipopolysaccharide. Int Immunopharmacol. 2010;10(3):331–8.
Tokaç M, Taner G, Aydın S, Özkardeş AB, Dündar HZ, Taşlıpınar MY, et al. Protective effects of curcumin against oxidative stress parameters and DNA damage in the livers and kidneys of rats with biliary obstruction. Food Chem Toxicol. 2013;61:28–35.
Ranjan A, Mukerjee A, Helson L, Vishwanatha J. Scale up, optimization and stability analysis of Curcumin C3 complex-loaded nanoparticles for cancer therapy. J Nanobiotechnol. 2012;10(1):38.
Basnet P, Hussain H, Tho I, Skalko-Basnet N. Liposomal delivery system enhances anti-inflammatory properties of curcumin. J Pharm Sci. 2012;101(2):598–609.
Liu JS, Xu LH, Liu CT, Zhang DF, Wang SG, Deng ZN, et al. Preparation and characterization of cationic curcumin nanoparticles for improvement of cellular uptake. Carbohydr Polym. 2012;90(1):16–22.
Mourtas S, Lazar AN, Markoutsa E, Duyckaerts C, Antimisiaris SG. Multifunctional nanoliposomes with curcumin–lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur J Med Chem. 2014;80:175–83.
Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, et al. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79(3):330–8.
Nair KL, Thulasidasan AKT, Deepa G, Anto RJ, Kumar GSV. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425:44–52.
Ma Z, Haddadi A, Molavi O, Lavasanifar A, Lai R, Samuel J. Micelles of poly(ethylene oxide)-b-poly(ε-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res A. 2008;86A(2):300–10.
Cheng K, Yeung C, Ho S, Chow S, Chow AL, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood–brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15(2):324–36.
Jain S, Amiji M. Tuftsin-modified alginate nanoparticles as a noncondensing macrophage-targeted DNA delivery system. Biomacromolecules. 2012;13(4):1074–85.
Manju S, Sreenivasan K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone–curcumin conjugate. J Pharm Sci. 2011;100(2):504–11.
Yen F-L, Wu T-H, Tzeng C-W, Lin L-T, Lin C-C. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhance its antioxidant and antihepatoma activities. J Agric Food Chem. 2010;58(12):7376–82.
Gangwar RK, Dhumale VA, Kumari D, Nakate UT, Gosavi SW, Sharma RB, et al. Conjugation of curcumin with PVP capped gold nanoparticles for improving bioavailability. Mater Sci Eng C. 2012;32(8):2659–63.
Masuda T, Hidaka K, Shinohara A, Maekawa T, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J Agric Food Chem. 1998;47(1):71–7.
Shen L, Ji H-F. Theoretical study on physicochemical properties of curcumin. Spectrochim Acta A. 2007;67(3–4):619–23.
Tang B, Ma L, Wang H-y, Zhang G-y. Study on the supramolecular interaction of curcumin and β-cyclodextrin by spectrophotometry and its analytical application. J Agric Food Chem. 2002;50(6):1355–61.
Bernabé-Pineda M, Ramírez-Silva Ma T, Romero-Romo M, González-Vergara E, Rojas-Hernández A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim Acta A. 2004;60(5):1091–7.
Wang Z, Leung M, Kee T, English D. The role of charge in the surfactant-assisted stabilization of the natural product curcumin. Langmuir. 2009;26:7.
Song KC, Lee HS, Choung IY, Cho KI, Ahn Y, Choi EJ. The effect of type of organic phase solvents on the particle size of poly(d,l-lactide-co-glycolide) nanoparticles. Colloids Surf A. 2006;276(1鈥?):162–7.
Schärtl W. Light scattering from polymer solutions and nanoparticle dispersions. Germany: Springer; 2007.
Karavas E, Georgarakis M, Docoslis A, Bikiaris D. Combining SEM, TEM, and micro-Raman techniques to differentiate between the amorphous molecular level dispersions and nanodispersions of a poorly water-soluble drug within a polymer matrix. Int J Pharm. 2007;340(1–2):76–83.
Easo SL, Mohanan PV. Dextran stabilized iron oxide nanoparticles: synthesis, characterization and in vitro studies. Carbohydr Polym. 2013;92(1):726–32.
Abdelwahed W, Degobert G, Fessi H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur J Pharm Biopharm. 2006;63(2):87–94.
Takeuchi H, Yamamoto H, Toyoda T, Toyobuku H, Hino T, Kawashima Y. Physical stability of size controlled small unilameller liposomes coated with a modified polyvinyl alcohol. Int J Pharm. 1998;164(1鈥?):103–11.
Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15(4):897–900.
Bhattacharjee S, de Haan L, Evers N, Jiang X, Marcelis A, Zuilhof H, et al. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part Fibre Toxicol. 2010;7(1):25.
Hoskins C, Cuschieri A, Wang L. The cytotoxicity of polycationic iron oxide nanoparticles: common endpoint assays and alternative approaches for improved understanding of cellular response mechanism. J Nanobiotechnol. 2012;10(1):15.
Cho EC, Xie J, Wurm PA, Xia Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009;9(3):1080–4.
Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun. 2007;353(1):26–32.
Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. Small. 2010;6(1):12–21.
Laroui H, Theiss AL, Yan Y, Dalmasso G, Nguyen HTT, Sitaraman SV, et al. Functional TNFα gene silencing mediated by polyethyleneimine/TNFα siRNA nanocomplexes in inflamed colon. Biomaterials. 2011;32(4):1218–28.
Matthäus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem. 2002;50(12):3444–52.
Barnes PJ. Nitric oxide and airway disease. Ann Med. 1995;27(3):389–93.
Andreasen SØ, Chong S-F, Wohl BM, Goldie KN, Zelikin AN. Poly(vinyl alcohol) physical hydrogel nanoparticles, not polymer solutions, exert inhibition of nitric oxide synthesis in cultured macrophages. Biomacromolecules. 2013;14(5):1687–95.
Abe Y, Hashimoto SHU, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39(1):41–7.
Zhong F, Chen H, Han L, Jin Y, Wang W. Curcumin attenuates lipopolysaccharide-induced renal inflammation. Biol Pharm Bull. 2011;34(2):226–32.
Suzuki M, Betsuyaku T, Ito Y, Nagai K, Odajima N, Moriyama C, Nasuhara Y, Nishimura M. Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. 2009.
Meja KK, Rajendrasozhan S, Adenuga D, Biswas SK, Sundar IK, Spooner G, et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am J Respir Cell Mol Biol. 2008;39(3):312–23.
Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28(1):219–42.
ACKNOWLEDGMENTS AND DISCLOSURES
WH Lee is the recipient of Cancer Institute New South Wales (CINSW) Early Career Fellowship. PM Young is the recipient of an Australian Research Council Future Fellowship (project number FT110100996). D Traini is the recipient of an Australian Research Council Future Fellowship (project number FT12010063). The authors are grateful to Dr Lyn Moir for her assistance in conducting flow cytometry analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, WH., Loo, CY., Young, P.M. et al. Curcumin Nanoparticles Attenuate Production of Pro-inflammatory Markers in Lipopolysaccharide-Induced Macrophages. Pharm Res 33, 315–327 (2016). https://doi.org/10.1007/s11095-015-1789-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1789-9