Abstract
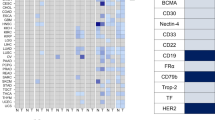
Antibody-drug conjugates (ADCs) represent a promising modality for the treatment of cancer. The therapeutic strategy is to deliver a potent drug preferentially to the tumor and not normal tissues by attaching the drug to an antibody that recognizes a tumor antigen. The selection of antigen targets is critical to enabling a therapeutic window for the ADC and has proven to be surprisingly complex. We surveyed the tumor and normal tissue expression profiles of the targets of ADCs currently in clinical development. Our analysis demonstrates a surprisingly broad range of expression profiles and the inability to formalize any optimal parameters for an ADC target. In this context, we discuss additional considerations for ADC target selection, including interdependencies among biophysical properties of the drug, biological functions of the target and strategies for clinical development. The TPBG (5T4) oncofetal antigen and the anti-TPBG ADC A1-mcMMAF are highlighted to demonstrate the relevance of the target’s biological function. Emerging platform technologies and novel biological insights are expanding ADC target space and transforming strategies for target selection.






Similar content being viewed by others
Abbreviations
- ADC:
-
Antibody-drug conjugate
- ALCL:
-
Anaplastic large-cell lymphoma
- APEX:
-
Absolute Protein Expression Measurements
- BRCA:
-
Breast invasive carcinoma
- CCLE:
-
Cancer Cell Line Encyclopedia
- CD:
-
Cluster of Differentiation
- CEACAM:
-
Carcinoembryonal antigen
- COAD:
-
Colon adenocarcinoma
- CSC:
-
Cancer stem cell
- DLBC:
-
Diffuse large B-cell lymphoma
- DLT:
-
Dose-limiting toxicity
- EBV:
-
Epstein-Barr Virus
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial-mesenchymal transition
- ERBB2:
-
erb-b2 receptor tyrosine kinase 2 (also known as HER2)
- G2/M:
-
Gap2 / mitosis
- GPI:
-
Glycosylphosphatidylinositol
- GTEx:
-
Genotype-Tissue Expression database
- HL:
-
Hodgkin’s lymphoma
- HSC:
-
Hematopoietic stem cell
- iBAQ:
-
Intensity-Based Absolute Quantification
- KIRC:
-
Kidney renal clear cell carcinoma
- LAML:
-
Acute myeloid leukemia
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- mAb:
-
Monoclonal antibody
- MESO:
-
Mesothelioma
- MMAF:
-
Monomethylauristatin F
- MTI:
-
Microtubule inhibitor
- NSCLC:
-
Non-small cell lung cancer
- OV:
-
Ovarian serous cystadenocarcinoma
- PAAD:
-
Pancreatic adenocarcinoma
- PDX:
-
Patient-derived xenograft
- PRAD:
-
Prostate adenocarcinoma
- PSMA:
-
Prostate-specific membrane antigen (also known as FOLH1)
- RPKM:
-
Reads per kilobase per million
- SKCM:
-
Skin Cutaneous Melanoma
- TCGA:
-
The Cancer Genome Atlas
- T-DM1:
-
Trastuzumab emtansine
- TIC:
-
Tumor-initiating cell
- TMDD:
-
Target-mediated drug disposition
- TPBG:
-
Trophoblast glycoprotein (also known as 5T4)
- TPM:
-
Transcripts per million
References
Krall N, Scheuerman J, Neri D. Small targeted cytotoxics: current state and promises from DNA-encoded chemical libraries. Angew Chem Int Ed. 2013;52:1384–402.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. NEJM. 2012;367(19):1783–91.
Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of Trastuzumab emtansine versus Trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J Clin Oncol. 2013;31(9):1157–63.
Mullard A. Maturing antibody-drug conjugate pipeline hits 30. Nat Rev Drug Discov. 2013;12:329–32.
Sapra P, Hooper AT, O’Donnell CJ, Gerber HP. Investigational antibody drug conjugates for solid tumors. Expert Opin Investig drugs. 2011;20:1131–49.
Burris HA, Gordon MS, Gerber DE, Spigel DR, Mendelson DS, Schiller JH, et al. A Phase 1 study of DNIB0600A, an Antibody-Drug Conjugate (ADC) Targeting NaPi2b, in Patients (Pts) with Non-Small Cell Lung Cancer (NSCLC) or Platinum-Resistant Ovarian Cancer (OC). J Clin Oncol 2014, 32:5s Supplement, Abstract 2504.
Sapra P, Damelin M, Dijoseph J, Marquette K, Geles KG, Golas J, et al. Long-term tumor regression induced by an antibody-drug conjugate that targets 5T4, an oncofetal antigen expressed on tumor-initiating cells. Mol Cancer Ther. 2013;12(1):38–47. doi:10.1158/1535-7163.MCT-12-0603.
Cancer Genome Atlas Research Network, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113–20. doi:10.1038/ng.2764.
GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–5.
Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63.
Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337–42.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7. doi:10.1038/nature11003. Erratum in: Nature. 2012 Dec 13;492(7428):290.
Polson AG, Yu SF, Elkins K, Zheng B, Clark S, Ingle GS, et al. Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood. 2007;110:616–23. doi:10.1182/blood-2007-01-066704.
Gerber DE, Infante JR, Gordon MS, Schiller JH, Spigel D, Wang Y et al. Safety, Pharmacokinetics, and Activity of the Anti-NaPi2b Antibody-Drug Conjugate DNIB0600A: A Phase I Study in Patients with Non-Small Cell Lung Cancer and Platinum-Resistant Ovarian Cancer. IASLC World Lung, Sydney, Australia, Oct 27–30, 2013.
Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, et al. A draft map of the human proteome. Nature. 2014;509:575–81.
Strassberger V, Trussel S, Fugmann T, Neri D, Roesli C. A novel reactive ester derivative of biotin with reduced membrane permeability for in vivo biotinylation experiments. Proteomics. 2010;10(19):3544–8.
Zhang H, Li XJ, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazine chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol. 2003;21(6):660–6.
Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982;93(1):97–102.
Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72.
Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborksa A, et al. The quantitative proteome of a human cell line. Mol Syst Biol. 2011;7:549.
Lu P, Vogel C, Wang R, Yao X, Marcotte M. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat Biotechnol. 2007;25(1):117–24.
Danila DC, Szmuelwitz RZ, Baron AD, Higano CS, Scher HI, Morris MJ, et al. A Phase I Study of DSTP3086S, an Antibody-Drug Conjugate (ADC) targeting STEAP-1, in Patients (Pts) with Metastatic Castration-Resitant Prostate Cancer (CRPC). J Clin Oncol 2014; 32:5s Suppl; Abstract 5024.
Burris HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate Trastuzumab-DM1 for the treatment of human epidermal growth factor receptor (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29(4):298–405.
Krop IE, LoRusso P, Miller KD, Modi S, Yardley D, Rodriguez G, et al. A phase II study of Trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer Who were previously treated with Trastuzumab, Lapatinib, an anthracycline, a taxane and capecitabine. J Clin Oncol. 2012;30(26):3234–41.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12.
Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240–5.
Fromm JR, McEarchern JA, Kennedy D, Thomas A, Shustov AR, Gopal AK. Clinical binding properties, internalization kinetics, and clinicopathologic activity of brentuximab vedotin: an antibody-drug conjugate for CD30-positive lymphoid neoplasms. Clin Lymhoma Myeloma Leuk. 2012;12(4):280–3.
Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9. doi:10.1200/JCO.2011.38.0410.
Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–6. doi:10.1200/JCO.2011.38.0402.
Tijink BM, Buter J, de Bree R, Giaccone G, Lang MS, Staab A, et al. A phase IDose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12(20):6064–72. doi:10.1158/1078-0432.CCR-06-0910.
Boswell CA, Mundo EE, Firestein R, Zhang C, Mao W, Gill H, et al. An integrated approach toidentify normal tissue expression of targets for antibody-drug conjugates: case study of TENB2. Br J Pharm. 2013;168:445–57. doi:10.1111/j.1476-5381.2012.02138.x.
Perrino E, Steiner M, Krall N, Bernardes GJ, Pretto F, Casi G, et al. Curative properties of noninternalizing antibody–drug conjugates based on maytansinoids. Cancer Res. 2014;74(9):2569–78. doi:10.1158/0008-5472.CAN-13-2990.
Ingle GS, Chan P, Elliott JM, Chang WS, Koeppen H, Stephan JP, et al. High CD21 expression inhibits internalization of anti-CD19 antibodies and cytotoxicity of an anti-CD19-drug conjugate. Br J Haematol. 2008;140(1):46–58.
Shi F, Sottile J. Caveolin-1-dependent 1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci. 2008;121:2360–71.
Muro S, Mateescu M, Gajewski C, Robinson M, Muzykantov VR, Koval M. Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am J Physiol Lung Cell Mol Physiol. 2006;290:L809–17. doi:10.1152/ajplung.00311.2005.
Du J, Chen X, Liang X, Zhang G, Xu J, He L, et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci. 2011;108(23):9466–71.
Teicher BA. Antibody-drug conjugate targets. Curr Cancer Drug Targets. 2009;9(8):982–1004.
Golfier S, Kopitz C, Kahnert A, Heisler I, Schatz CA, Stelte-Ludwig B, et al. Anetumab ravtansine - a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther. 2014;13(6):1537–48.
Govindan SV, Cardillo TM, Moon SJ, Hansen HJ, Goldenberg DM. CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res. 2009;15(19):6052–61. doi:10.1158/1078-0432.CCR-09-0586.
Kelly RK, Olson DL, Sun Y, Wen D, Wortham KA, Antognetti G, et al. An antibody-cytotoxic conjugate, BIIB015, is a new targeted therapy for Cripto positive tumours. Eur J Cancer. 2011;47(11):1736–46. doi:10.1016/j.ejca.2011.02.023.
Sausville E, LoRusso P, Quinn M, Forman K, Leamon C, Morganstern D, et al. A phase I study of EC145 administered weeks 1 and 3 of a 4-week cycle in patients with refractory solid tumors. J Clin Oncol. 2007;25(18S):2577.
Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–87.
Phillips GD, Fields CT, Li G, Dowbenko D, Schaefer G, Miller K, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res. 2014;20(2):456–68. doi:10.1158/1078-0432.CCR-13-0358.
Gwin WR, Spector NL. Pertuzumab protects the achilles’ heel of trastuzumab–emtansine. Clin Cancer Res. 2014;20(2):278–80. doi:10.1158/1078-0432.CCR-13-2626.
Tan X, Lu B, Jin G, Wang F, Myers J, Musto S, et al. Antibody-drug conjugates with modified linker-payloads overcome resistance to a trastuzumab-maytansinoid conjugate in multiple cultured tumor cell models. AACR Annual Meeting 2014, Abstract #1830.
Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006;17:114–24.
Sapra P, Stein R, Pickett J, Qu Z, Govindan SV, Cardillo TM, et al. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res. 2005;11(14):5257–64.
Laurent-Puig P, Lievre A, Blons H. Mutations and response to epidermal growth factor receptor inhibitors. Clin Cancer Res. 2009;15(4):1133–9. doi:10.1158/1078-0432.CCR-08-0905.
Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8(10):806–23. doi:10.1038/nrd2137.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer and cancer stem cells. Nature. 2001;414:105–11.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumors: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68.
Gerber HP, Senter PD, Grewal IS. Antibody drug-conjugates targeting the tumor vasculature: current and future developments. mAbs. 2009;1(3):247–53.
Ostermann E, Garin-Chesa P, Heider KH, Kalat M, Lamche H, Puri C, et al. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res. 2008;14(14):4584–92. doi:10.1158/1078-0432.CCR-07-5211.
Bernardes GJ, Casi G, Trussel S, Hartmann I, Schwager K, Scheuermann J, et al. A traceless vascular-targeting antibody-drug conjugate for cancer therapy. Angew Chem Int Ed Engl. 2012;51(4):941–4.
Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192–8.
Gutbrodt KL, Schliemann C, Giovannoni L, Frey K, Pabst T, Klapper W, et al. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med. 2013;5(201):201ra118. doi:10.1126/scitranslmed.3006221.
Sauer S, Erba PA, Petrini M, Menrad A, Giovannoni L, Grana C, et al. Expression of the oncofetal ED-B-containing fibronectin isoform in hematologic tumors enables ED-B-targeted 131I-L19SIP radioimmunotherapy in Hodgkin lymphoma patients. Blood. 2009;113(10):2265–74. doi:10.1182/blood-2008-06-160416.
Chen Y, Clark S, Wong T, Chen Y, Chen Y, Dennis MS, et al. Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, Are highly efficacious in animal tumor models. Cancer Res. 2007;67:4924–32.
Pak Y, Zhang Y, Pastan I, Lee B. Antigen shedding May improve efficiencies for delivery of antibody-based anticancer agents in solid tumors. Cancer Res. 2012;72:3143–52.
Shih SC, Sloper-Mould KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 2000;19:187–98.
Wright Jr GL, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48(2):326–34.
Ma D, Hopf CE, Malewicz AD, Donovan GP, Senter PD, Goeckeler WF, et al. Potent antitumor activity of an auristatin-conjugated, fully human monoclonal antibody to prostate-specific membrane antigen. Clin Cancer Res. 2006;12(8):2591–6.
Bander NH. Antibody-drug conjugate target selection: critical factors. Methods Mol Biol. 2013;1045:29–40.
Amato RJ, Stepankiw M. Evaluation of MVA-5 T4 as a novel immunotherapeutic vaccine in colorectal, renal and prostate cancer. Future Oncol. 2012;8(3):231–7. doi:10.2217/fon.12.7.
Forsberg G, Skartved NJ, Wallén-Ohman M, Nyhlén HC, Behm K, Hedlund G, et al. Naptumomab estafenatox, an engineered antibody-superantigen fusion protein with low toxicity and reduced antigenicity. J Immunother. 2010;33(5):492–9. doi:10.1097/CJI.0b013e3181d75820.
Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57:239–46.
Hole N, Stern PL. Isolation and characterization of 5T4, a tumour-associated antigen. Int J Cancer. 1990;45:179–84.
Barrow KM, Ward CM, Rutter J, Ali S, Stern PL. Embryonic expression of murine 5T4 oncofoetal antigen is associated with morphogenetic events at implantation and in developing epithelia. Dev Dyn. 2005;233(4):1535–45.
Damelin M, Geles KG, Follettie MT, Yuan P, Baxter M, Golas J, et al. Delineation of a cellular hierarchy in lung cancer reveals an oncofetal antigen expressed on tumor-initiating cells. Cancer Res. 2011;71:4236–46.
Naganuma H, Kono K, Mori Y, Takayoshi S, Stern PL, Tasaka K, et al. Oncofetal antigen 5T4 expression as a prognostic factor in patients with gastric cancer. Anticancer Res. 2002;22(2B):1033–8.
Starzynska T, Marsh PJ, Schofield PF, Roberts SA, Myers KA, Stern PL. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69(5):899–902.
Wrigley E, McGown AT, Rennison J, Swindell R, Crowther D, Starzynska T, et al. 5T4 oncofetal antigen expression in ovarian carcinoma. Int J Gynecol Cancer. 1995;5(4):269–74.
Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, Stern PL, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67(23):11254–62.
Spencer HL, Eastham AM, Merry CL, Southgate TD, Perez-Campo F, Soncin F, et al. E-cadherin inhibits cell surface localization of the pro-migratory 5T4 oncofetal antigen in mouse embryonic stem cells. Mol Biol Cell. 2007;18(8):2838–51.
Carsberg CJ, Myers KA, Stern PL. Metastasis-associated 5T4 antigen disrupts cell-cell contacts and induces cellular motility in epithelial cells. Int J Cancer. 1996;68(1):84–92.
Kagermeier-Schenk B, Wehner D, Ozhan-Kizil G, Yamamoto H, Li J, Kirchner K, et al. Waif1/5T4 inhibits Wnt/β-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev Cell. 2011;21(6):1129–43. doi:10.1016/j.devcel.2011.10.015.
Gromova P, Ralea S, Lefort A, Libert F, Rubin BP, Erneux C, et al. Kit K641E oncogene up-regulates Sprouty homolog 4 and trophoblast glycoprotein in interstitial cells of Cajal in a murine model of gastrointestinal stromal tumours. J Cell Mol Med. 2009;13(8A):1536–48. doi:10.1111/j.1582-4934.2009.00768.x.
Southgate TD, McGinn OJ, Castro FV, Rutkowski AJ, Al-Muftah M, Marinov G, et al. CXCR4 mediated chemotaxis is regulated by 5T4 oncofetal glycoprotein in mouse embryonic cells. PLoS ONE. 2010;5(4):e9982. doi:10.1371/journal.pone.0009982.
McGinn OJ, Marinov G, Sawan S, Stern PL. CXCL12 receptor preference, signal transduction, biological response and the expression of 5T4 oncofoetal glycoprotein. J Cell Sci. 2012;125(Pt 22):5467–78. doi:10.1242/jcs.109488.
Sagert J, West J, Wong C, Desnoyers L, Vasiljeva O, Richardson J, et al. Transforming Notch ligands into tumor-antigen targets: a Probody-Drug Conjugate (PDC) targeting Jagged 1 and Jagged 2. AACR Annual Meeting, 2014, Abstract #2665.
Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–32. doi:10.1038/nbt.1480.
Panowksi S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. mAbs. 2014;6(1):34–45. doi:10.4161/mabs.27022.
Strop P, Liu SH, Dorywalska M, Delaria K, Dushin RG, Tran TT, et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol. 2013;20:161–7.
Thomas J, Yurkovetskiy A, Bodyak N, et al. Polyacetal polymer-based anti-HER2 antibody-drug conjugate employing cysteine bioconjugation through thioether linkage allows a high drug loading of dolastatin-derived payload with excellent pharmacokinetics and potent anti-tumor activity. Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2013 Oct 19–23; Boston, MA. Philadelphia (PA): AACR; Mol Cancer Ther 2013; 12(11 Suppl): Abstract nr C238
Acknowledgments
The authors thank Eugene Melamud for assistance with the proteomics data analysis and Chris O’Donnell, Hans Peter Gerber, Kenneth Geles, Paul Rejto and Jeremy Barton for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Damelin, M., Zhong, W., Myers, J. et al. Evolving Strategies for Target Selection for Antibody-Drug Conjugates. Pharm Res 32, 3494–3507 (2015). https://doi.org/10.1007/s11095-015-1624-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1624-3