Abstract
Purpose
To predict the temperature-composition phase diagram of solid dispersions (SDs) through theoretical approaches using cinnarizine-Soluplus® SD as a model system and evaluate the predicted results.
Methods
A complete phase diagram of cinnarizine-Soluplus® SD, including the solubility curve, miscibility curve and glass transition temperature curve, was constructed on the basis of the solid–liquid equilibrium (SLE) equation, Florry-Huggins (F-H) theory and Fox equation. Cinnarizine-Soluplus® SDs with different drug loadings were prepared by hot melt extrusion. The extrudates and corresponding physical mixtures were analyzed to check the predicted results.
Results
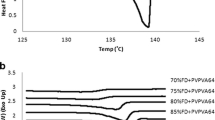
The experimental data revealed a solubility of 7.9 wt% at 110°C and a miscibility level of 65 wt% at room temperature, which were both consistent with predicted values.
Conclusions
The predicted phase diagram agrees well with the experimental results for the non-polar components which mainly interact through dispersion forces, thus facilitating the formulation design of SDs.












Similar content being viewed by others
References
Alam MA, Ali R, Al-Jenoobi FI, Al-Mohizea AM. Solid dispersions: a strategy for poorly aqueous soluble drugs and technology updates. Expert Opin Drug Deliv. 2012;9(11):1419–40.
Qian F, Huang J, Hussain MA. Drug–polymer solubility and miscibility: stability consideration and practical challenges in amorphous solid dispersion development. J Pharm Sci. 2010;99(7):2941–7.
Zhao Y, Inbar P, Chokshi HP, Malick AW, Choi DS. Prediction of the thermal phase diagram of amorphous solid dispersions by Flory–Huggins theory. J Pharm Sci. 2011;100(8):3196–207.
Tao J, Sun Y, Zhang GG, Yu L. Solubility of small-molecule crystals in polymers: d-mannitol in PVP, indomethacin in PVP/VA, and nifedipine in PVP/VA. Pharm Res. 2009;26(4):855–64.
Karavas E, Georgarakis M, Docoslis A, Bikiaris D. Combining SEM, TEM, and micro-Raman techniques to differentiate between the amorphous molecular level dispersions and nanodispersions of a poorly water-soluble drug within a polymer matrix. Int J Pharm. 2007;340(1):76–83.
A.M. Kaushal, P. Gupta, and A.K. Bansal. Amorphous drug delivery systems: molecular aspects, design, and performance. Critical Reviews™ in Ther Drug Carrier Syst. 2004;21(3).
Greco S, Authelin J-R, Leveder C, Segalini A. A practical method to predict physical stability of amorphous solid dispersions. Pharm Res. 2012;29(10):2792–805.
Linand D, Huang Y. A thermal analysis method to predict the complete phase diagram of drug–polymer solid dispersions. Int J Pharm. 2010;399(1):109–15.
Sun Y, Tao J, Zhang GG, Yu L. Solubilities of crystalline drugs in polymers: an improved analytical method and comparison of solubilities of indomethacin and nifedipine in PVP, PVP/VA, and PVAc. J Pharm Sci. 2010;99(9):4023–31.
Mahieu A, Willart J-F, Dudognon E, Danède F, Descamps M. A new protocol to determine the solubility of drugs into polymer matrixes. Mol Pharm. 2013;10(2):560–6.
Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug–polymer miscibility and solubility. Pharm Res. 2006;23(10):2417–26.
Marsac PJ, Li T, Taylor LS. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26(1):139–51.
Bellantone RA, Patel P, Sandhu H, Choi DS, Singhal D, Chokshi H, et al. A method to predict the equilibrium solubility of drugs in solid polymers near room temperature using thermal analysis. J Pharm Sci. 2012;101(12):4549–58.
M. Rubinsteinand R.H. Colby. Polymer physics, OUP Oxford; 2003.
S.Z. Cheng. Phase transitions in polymers: the role of metastable states: the role of metastable states, Elsevier; 2008.
Kalogerasand IM, Brostow W. Glass transition temperatures in binary polymer blends. J Polym Sci B. 2009;47(1):80–95.
Jayachandra Babu R, Brostow W, Kalogeras IM, Sathigari S. Glass transitions in binary drug + polymer systems. Mater Lett. 2009;63(30):2666–8.
Kalogeras IM. A novel approach for analyzing glass-transition temperature vs. composition patterns: application to pharmaceutical compound + polymer systems. Eur J Pharm Sci. 2011;42(5):470–83.
A. Chan, K. Coppens, M. Hall, V. He, P. Jog, P. Larsen, B. Koblinksi, M. Read, D. Rothe, and S. Somasi. Solubility parameters as a tool to predict API morphology in hot melt extruded (HME) formulations containing ethycellulose, hypromellose, and polyethylene oxide, 2006 Am Assoc Pharm Sci Annu Meet Expo, San Antonio, TX, Vol. 29,2006.
Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K. Preparation of carbamazepine–Soluplus® solid dispersions by hot-melt extrusion, and prediction of drug–polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm. 2013;84(1):228–37.
Frankand TC, Gupta S. Quickly screen solvents for organic solids. Chem Eng Prog. 1999;95(12):41–61.
Van Eerdenbrugh B, Vermant J, Martens JA, Froyen L, Van Humbeeck J, Augustijns P, et al. A screening study of surface stabilization during the production of drug nanocrystals. J Pharm Sci. 2009;98(6):2091–103.
Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm. 2001;226(1):147–61.
Fox T. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull Am Phys Soc. 1956;1(123):22060–6218.
Gramaglia D, Conway BR, Kett VL, Malcolm RK, Batchelor HK. High speed DSC (hyper-DSC) as a tool to measure the solubility of a drug within a solid or semi-solid matrix. Int J Pharm. 2005;301(1):1–5.
Ogata H, Aoyagi N, Kaniwa N, Ejima A, Sekine N, Kitamura M, et al. Gastric acidity dependent bioavailability of cinnarizine from two commercial capsules in healthy volunteers. Int J Pharm. 1986;29(2):113–20.
Tian Y, Booth J, Meehan E, Jones DS, Li S, Andrews GP. Construction of drug–polymer thermodynamic phase diagrams using Flory–Huggins interaction theory: identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol Pharm. 2012;10(1):236–48.
Rimand PB, Runt JP. Melting point depression in crystalline/compatible polymer blends. Macromolecules. 1984;17(8):1520–6.
Nair R, Nyamweya N, Gönen S, Martınez-Miranda L, Hoag S. Influence of various drugs on the glass transition temperature of poly (vinylpyrrolidone): a thermodynamic and spectroscopic investigation. Int J Pharm. 2001;225(1):83–96.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, B., Wang, X., Zhang, Y. et al. Theoretical Prediction of a Phase Diagram for Solid Dispersions. Pharm Res 32, 840–851 (2015). https://doi.org/10.1007/s11095-014-1500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1500-6