Abstract
Purpose
Early identification of monoclonal antibody candidates whose development, as high concentration (≥100 mg/mL) drug products, could prove challenging, due to high viscosity, can help define strategies for candidate engineering and selection.
Methods
Concentration dependent viscosities of 11 proprietary mAbs were measured. Sequence and structural features of the variable (Fv) regions were analyzed to understand viscosity behavior of the mAbs. Coarse-grained molecular simulations of two problematic mAbs were compared with that of a well behaved mAb.
Results
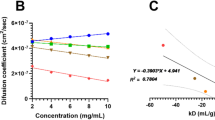
Net charge, ξ-potential and pI of Fv regions were found to correlate with viscosities of highly concentrated antibody solutions. Negative net charges on the Fv regions of two mAbs with poor viscosity behaviors facilitate attractive self-associations, causing them to diffuse slower than a well-behaved mAb with positive net charge on its Fv region. An empirically derived equation that connects aggregation propensity and pI of the Fv region with high concentration viscosity of the whole mAb was developed.
Conclusions
An Fv region-based qualitative screening profile was devised to flag mAb candidates whose development, as high concentration drug products, could prove challenging. This screen can facilitate developability risk assessment and mitigation strategies for antibody based therapeutics via rapid high throughput material-free screening.






Similar content being viewed by others
Abbreviations
- <x 2>:
-
Average mean squared displacement
- CDR:
-
Complementarity determining region
- CH1:
-
First domain in constant portion of a heavy chain
- CH2:
-
Second domain in constant portion of a heavy chain
- CH3:
-
Third domain in constant portion of a heavy chain
- cIEF:
-
Capillary Iso-Electric Focusing
- cP:
-
Centi Poise
- D:
-
Diffusion coefficient
- DFv :
-
Dipole moment for Fv portion of a mAb
- Dwhole mAb :
-
Dipole moment of a full length mAb
- Fab:
-
Fragment antigen binding
- Fc:
-
Fragment crystallize-able
- Fv:
-
Fragment variable
- GB/VI:
-
Generalized Born/Volume Integral
- IgG:
-
Immunoglobulin G
- kB :
-
Boltzmann constant
- KD hydromomFv :
-
Kyte-Doolittle hydrophobicity moment for Fv portion of a mAb
- LAMMPS:
-
Large-scale Atomic/Molecular Massively Parallel Simulator
- mAb:
-
Monoclonal antibody
- MOE:
-
Molecular Operations Environment
- NormASAhphob:
-
Normalized hydrophobic surface area
- NresFv :
-
Number of residues in the Fv region
- PaggTANGOFv :
-
Normalized aggregation propensity of an Fv region computed by using TANGO
- PaggWaltzFv :
-
Normalized aggregation propensity of an Fv region computed by using Waltz
- pIFv :
-
Isoelectric point for Fv portion of a mAb
- pIwhole mAb :
-
Isoelectric point of a full length mAb
- RMSE:
-
Root Mean Square Error
- RMSG:
-
Root Mean Square Gradient
- rpm:
-
Revolutions per minute
- UF/DF:
-
Ultra-Filtration/Dia-Filtration
- UV–vis:
-
Ultraviolet–visible
- VH :
-
Variable domain of a heavy chain
- VL :
-
Variable domain of a light chain
- Zapp whole mAb :
-
Apparent charge on a full length mAb
- ZappFv :
-
Apparent charge on Fv portion
- Zconstant regions :
-
Net charge on the constant regions of a mAb
- ZFv :
-
Net charge on Fv portion of a mAb
- Zwhole mAb :
-
Net charge on a full length mAb
- η:
-
Measured viscosity of an antibody solution
- ηrel :
-
Relative viscosity of an antibody
- ηsp :
-
Specific viscosity of an antibody
- μ:
-
Particle mobility
- ξconstant regions :
-
Zeta potential of constant regions of a mAb
- ξFv :
-
Zeta potential of Fv portion of a mAb
- ξ-potential:
-
Zeta potential
- ξwhole mAb :
-
Zeta potential of a full length mAb
References
Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005;94:1928–40.
Johnsonand HR, Lenhoff AM. Characterization and Suitability of Therapeutic Antibody Dense Phases for Subcutaneous Delivery. Mol Pharm 2013.
Galush WJ, Le LN, Moore JM. Viscosity behavior of high-concentration protein mixtures. J Pharm Sci. 2012;101:1012–20.
Narasimhan C, Mach H, Shameem M. High-dose monoclonal antibodies via the subcutaneous route: challenges and technical solutions, an industry perspective. Ther Deliv. 2012;3:889–900.
Jezek J, Rides M, Derham B, Moore J, Cerasoli E, Simler R, et al. Viscosity of concentrated therapeutic protein compositions. Adv Drug Deliv Rev. 2011;63:1107–17.
Cromwell ME, Hilario E, Jacobson F. Protein aggregation and bioprocessing. AAPS J. 2006;8:E572–9.
Lilyestrom WG, Yadav S, Shire SJ, Scherer TM. Monoclonal antibody self-association, cluster formation, and rheology at high concentrations. J Phys Chem B. 2013;117:6373–84.
Guo Z, Chen A, Nassar RA, Helk B, Mueller C, Tang Y, et al. Structure-activity relationship for hydrophobic salts as viscosity-lowering excipients for concentrated solutions of monoclonal antibodies. Pharm Res. 2012;29:3102–9.
Kamerzell TJ, Pace AL, Li M, Danilenko DM, McDowell M, Gokarn YR, et al. Polar solvents decrease the viscosity of high concentration IgG1 solutions through hydrophobic solvation and interaction: formulation and biocompatibility considerations. J Pharm Sci. 2013;102:1182–93.
Srinivasan C, Weight AK, Bussemer T, Klibanov AM. Non-aqueous suspensions of antibodies are much less viscous than equally concentrated aqueous solutions. Pharm Res. 2013;30:1749–57.
He F, Becker GW, Litowski JR, Narhi LO, Brems DN, Razinkov VI. High-throughput dynamic light scattering method for measuring viscosity of concentrated protein solutions. Anal Biochem. 2010;399:141–3.
Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JM, et al. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J. 2012;103:69–78.
He F, Woods CE, Trilisky E, Bower KM, Litowski JR, Kerwin BA, Becker GW, Narhi LO, Razinkov VI. Screening of monoclonal antibody formulations based on high-throughput thermostability and viscosity measurements: Design of experiment and statistical analysis. J Pharm Sci 2010.
Agrawal NJ, Kumar S, Wang X, Helk B, Singh SK, Trout BL. Aggregation in protein-based biotherapeutics: computational studies and tools to identify aggregation-prone regions. J Pharm Sci. 2011;100:5081–95.
Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Design of therapeutic proteins with enhanced stability. Proc Natl Acad Sci U S A. 2009;106:11937–42.
Wang X, Das TK, Singh SK, Kumar S. Potential aggregation prone regions in biotherapeutics: a survey of commercial monoclonal antibodies. MAbs. 2009;1:254–67.
Wang X, Singh SK, Kumar S. Potential aggregation-prone regions in complementarity-determining regions of antibodies and their contribution towards antigen recognition: a computational analysis. Pharm Res. 2010;27:1512–29.
Wang X, Kumar S, Buck PM, Singh SK. Impact of deglycosylation and thermal stress on conformational stability of a full length murine IgG2a monoclonal antibody: Observations from molecular dynamics simulations. Proteins. 2013;81:443–60.
Buck PM, Kumar S, Wang X, Agrawal NJ, Trout BL, Singh SK. Computational methods to predict therapeutic protein aggregation. Methods Mol Biol. 2012;899:425–51.
Buck PM, Kumar S, Singh SK. Insights into the potential aggregation liabilities of the b12 Fab fragment via elevated temperature molecular dynamics. Protein Eng Des Sel. 2013;26:195–206.
Buck PM, Kumar S, Singh SK On the role of aggregation prone regions in protein evolution, stability, and enzymatic catalysis: insights from diverse analyses. PLoS Comp Biol. In press: 2013.
Yadav S, Laue TM, Kalonia DS, Singh SN, Shire SJ. The influence of charge distribution on self-association and viscosity behavior of monoclonal antibody solutions. Mol Pharm. 2012;9:791–802.
Yadav S, Sreedhara A, Kanai S, Liu J, Lien S, Lowman H, et al. Establishing a link between amino acid sequences and self-associating and viscoelastic behavior of two closely related monoclonal antibodies. Pharm Res. 2011;28:1750–64.
Harn N, Allan C, Oliver C, Middaugh CR. Highly concentrated monoclonal antibody solutions: direct analysis of physical structure and thermal stability. J Pharm Sci. 2007;96:532–46.
Kamerzell TJ, Kanai S, Liu J, Shire SJ, Wang YJ. Increasing IgG concentration modulates the conformational heterogeneity and bonding network that influence solution properties. J Phys Chem B. 2009;113:6109–18.
Neergaard MS, Kalonia DS, Parshad H, Nielsen AD, Moller EH, van de Weert M. Viscosity of high concentration protein formulations of monoclonal antibodies of the IgG1 and IgG4 subclass - prediction of viscosity through protein-protein interaction measurements. Eur J Pharm Sci. 2013;49:400–10.
Chaudhri A, Zarraga IE, Kamerzell TJ, Brandt JP, Patapoff TW, Shire SJ, et al. Coarse-grained modeling of the self-association of therapeutic monoclonal antibodies. J Phys Chem B. 2012;116:8045–57.
Chaudhri A, Zarraga IE, Yadav S, Patapoff TW, Shire SJ, Voth GA. The role of amino acid sequence in the self-association of therapeutic monoclonal antibodies: insights from coarse-grained modeling. J Phys Chem B. 2013;117:1269–79.
Yadav S, Shire SJ, Kalonia DS. Viscosity behavior of high-concentration monoclonal antibody solutions: correlation with interaction parameter and electroviscous effects. J Pharm Sci. 2012;101:998–1011.
Laue T. Proximity energies: a framework for understanding concentrated solutions. J Mol Recognit. 2012;25:165–73.
Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci. 2010;99:4812–29.
Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci. 2010;99:1152–68.
Saito S, Hasegawa J, Kobayashi N, Kishi N, Uchiyama S, Fukui K. Behavior of monoclonal antibodies: relation between the second virial coefficient (B (2)) at low concentrations and aggregation propensity and viscosity at high concentrations. Pharm Res. 2012;29:397–410.
Warne NW. Development of high concentration protein biopharmaceuticals: the use of platform approaches in formulation development. Eur J Pharm Biopharm. 2011;78:208–12.
Bolton GR, Boesch AW, Basha J, Lacasse DP, Kelley BD, Acharya H. Effect of protein and solution properties on the Donnan effect during the ultrafiltration of proteins. Biotechnol Prog. 2011;27:140–52.
Creighton TE The physical and chemical basis of molecular biology, Helvetian Press, 2010.
Saphire EO, Stanfield RL, Crispin MD, Morris G, Zwick MB, Pantophlet RA, et al. Crystal structure of an intact human IgG: antibody asymmetry, flexibility, and a guide for HIV-1 vaccine design. Adv Exp Med Biol. 2003;535:55–66.
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The protein data bank. Nucleic Acids Res. 2000;28:235–42.
Fernandez-Escamilla AM, Rousseau F, Schymkowitz J, Serrano L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat Biotechnol. 2004;22:1302–6.
Maurer-Stroh S, Debulpaep M, Kuemmerer N, Lopez de la Paz M, Martins IC, Reumers J, et al. Exploring the sequence determinants of amyloid structure using position-specific scoring matrices. Nat Methods. 2010;7:237–42.
Mant CT, Kovacs JM, Kim HM, Pollock DD, Hodges RS. Intrinsic amino acid side-chain hydrophilicity/hydrophobicity coefficients determined by reversed-phase high-performance liquid chromatography of model peptides: comparison with other hydrophilicity/hydrophobicity scales. Biopolymers. 2009;92:573–95.
Plimpton SJ. Fast parallel algorithms for short-range molecular dynamics. J Comp Physiol. 1995;117:1–19.
Medhi J. Statistical methods: An introductory text. New Delhi: Wiley Eastern Limited; 1992.
Minton AP. Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations. J Pharm Sci. 2005;94:1668–75.
Mooney M. The viscosity of a concentrated suspension of spherical particles. J Colloid Sci. 1951;6:162–70.
Teplyakov A, Zhao Y, Malia TJ, Obmolova G, Gilliland GL. IgG2 Fc structure and the dynamic features of the IgG CH2-CH3 interface. Mol Immunol. 2013;56:131–9.
Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, et al. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol. 2014;426:630–44.
Ely KR, Herron JN, Harker M, Edmundson AB. Three-dimensional structure of a light chain dimer crystallized in water. Conformational flexibility of a molecule in two crystal forms. J Mol Biol. 1989;210:601–15.
Vincentand KJ, Zurini M. Current strategies in antibody engineering: Fc engineering and pH-dependent antigen binding, bispecific antibodies and antibody drug conjugates. Biotechnol J. 2012;7:1444–50.
Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, et al. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods. 2014;65:114–26.
Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody diffusion in human cervical mucus. Biophys J. 1994;66:508–15.
Miao L, Qin H, Koehl P, Song J. Selective and specific ion binding on proteins at physiologically-relevant concentrations. FEBS Lett. 2011;585:3126–32.
Gokarn YR, Fesinmeyer RM, Saluja A, Razinkov V, Chase SF, Laue TM, et al. Effective charge measurements reveal selective and preferential accumulation of anions, but not cations, at the protein surface in dilute salt solutions. Protein Sci. 2011;20:580–7.
Lehermayr C, Mahler HC, Mader K, Fischer S. Assessment of net charge and protein-protein interactions of different monoclonal antibodies. J Pharm Sci. 2011;100:2551–62.
Laue T. How solvent and excipient properties impact aggregation and viscosity. Palm Springs: CHI PepTalk; 2013.
Bas DC, Rogers DM, Jensen JH. Very fast prediction and rationalization of pKa values for protein-ligand complexes. Proteins. 2008;73:765–83.
Rostkowski M, Olsson MH, Sondergaard CR, Jensen JH. Graphical analysis of pH-dependent properties of proteins predicted using PROPKA. BMC Struct Biol. 2011;11:6.
Kieseritzkyand G, Knapp EW. Optimizing pKa computation in proteins with pH adapted conformations. Proteins. 2008;71:1335–48.
Acknowledgments and Disclosures
Pfizer Business Technology is thanked for computational facilities. A postdoctoral fellowship to P.M.B. by Pfizer Inc. is gratefully acknowledged. Drs. Donna Luisi, David Sek, Norman MacDougall and Robert Walters are acknowledged for several constructive discussions and help with experiments. S.K. acknowledges his discussions with Joseph McLaughlin on viscosity measurements and data manipulations. All authors are employees of Pfizer Inc.
LL, CB, PN, NL, DB and JL performed the experiments. SK performed data analyses and PMB performed Coarse-grained simulations. LL and SK wrote most of the manuscript. SK and SKS conceived the concept of using molecular modeling to understand viscosity. All authors read and contributed towards improving the manuscript draft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L., Kumar, S., Buck, P.M. et al. Concentration Dependent Viscosity of Monoclonal Antibody Solutions: Explaining Experimental Behavior in Terms of Molecular Properties. Pharm Res 31, 3161–3178 (2014). https://doi.org/10.1007/s11095-014-1409-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1409-0