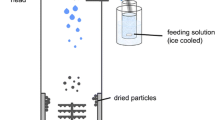
Abstract
Purpose
The aim of this study was to investigate the potential of using a spray-dryer equipped with a 3-fluid nozzle to microencapsulate protein drugs into polymeric microparticles.
Methods
Lysozyme and PLGA were used as a model protein and a model polymer, respectively. The effects of process and formulation variables, such as i) the type of organic solvent, ii) the feeding rate ratio of the outer PLGA-containing feed solution to inner lysozyme-containing feed solution, and iii) the mass ratio of PLGA to protein, on the properties (morphology, internal structure, protein surface enrichment and release profiles) of the spray dried microparticles were investigated to understand protein microencapsulation and particle formation mechanisms.
Results
The spherical, condensed microparticles were obtained with D50 of 1.07–1.60 μm and Span in the range of 0.82–1.23. The lysozyme surface content decreased upon different organic solvents used as follows: acetonitrile > acetone > dichloromethane. Additionally, the lysozyme surface enrichment decreased slightly when increasing the feeding rate ratio of the outer feed solution to the inner feed solution from 4:1 to 10:1. Furthermore, it was observed that there was a correlation between the degree of burst release and the lysozyme surface enrichment, whereas the lysozyme loading content had no substantial impact on the release kinetics.
Conclusions
The present work demonstrates the potential of spray dryer equipped with a 3-fluid nozzle in microencapsulation of proteins into PLGA matrices with different characteristics by varying process and formulation parameters.








Similar content being viewed by others
References
Yeo Y, Park K. A new microencapsulation method using an ultrasonic atomizer based on interfacial solvent exchange. J Control Release. 2004;100(3):379–88.
Gaozhong Z, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide). Nature Biotech. 2000;18:52–7.
Wu F, Jin T. Polymer-based sustained-release dosage forms for protein drugs, challenges, and recent advances. AAPS PharmSciTech. 2008;9(4):1218–29.
Desai KG, Schwendeman SP. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J Control Release. 2013;165(1):62–74.
Mok H, Park TG. Water-free microencapsulation of proteins within PLGA microparticles by spray drying using PEG-assisted protein solubilization technique in organic solvent. Eur J Pharm Biopharm. 2008;70(1):137–44.
Giteau A, Venierjulienne M, Marchal S, Courthaudon J, Sergent M, Monteromenei C, et al. Reversible protein precipitation to ensure stability during encapsulation within PLGA microspheres. Eur J Pharm Biopharm. 2008;70(1):127–36.
Fude C, Dongmei C, Anjin T, Mingshi Y, Kai S, Min Z, et al. Preparation and characterization of melittin-loaded poly (dl-lactic acid) or poly (dl-lactic-co-glycolic acid) microspheres made by the double emulsion method. J Control Release. 2005;107(2):310–9.
Wang J, Wang BM, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly(D, L-lactide-co-glycolide) microspheres. J Control Release. 2002;82(2–3):289–307.
Geng Y, Yuan W, Wu F, Chen J, He M, Jin T. Formulating erythropoietin-loaded sustained-release PLGA microspheres without protein aggregation. J Control Release. 2008;130(3):259–65.
Johansen P, Estevez F, Zurbriggen R, Merkle HP, Glück R, Corradin G, et al. Towards clinical testing of a single-administration tetanus vaccine based on PLA/PLGA microspheres. Vaccine. 2000;19:8.
Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364(2):298–327.
Giteau A, Venier-Julienne MC, Aubert-Pouëssel A, Benoit JP. How to achieve sustained and complete protein release from PLGA-based microparticles? Int J Pharm. 2008;350(1–2):14–26.
Jiang W, Schwendeman SP. Stabilization of tetanus toxoid encapsulated in PLGA microspheres. Mol Pharm. 2008;5(5):808–17.
Katare YK, Panda AK. Influences of excipients on in vitro release and in vivo performance of tetanus toxoid loaded polymer particles. Eur J Pharm Sci. 2006;28(3):179–88.
Yang M. Spray drying pharmaceuticals. Eur Pharm Review. 2011;16(6):64–8.
Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022.
Sollohub K, Cal K. Spray drying technique: II. Current applications in pharmaceutical technology. J Pharm Sci. 2010;99(2):587–97.
Lebrun P, Krier F, Mantanus J, Grohganz H, Yang M, Rozet E, et al. Design space approach in the optimization of the spray-drying process. Eur J Pharm Biopharm. 2012;80(1):226–34.
White S, Bennett DB, Cheu S, Conley PW, Guzek DB, Gray S, et al. EXUBERA: pharmaceutical development of a novel product for pulmonary delivery of insulin. Diabetes Technol Ther. 2005;7(6):896–906.
Tsapis N, Bennett D, Jackson B, Weitz DA, Edwards DA. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci U S A. 2002;99(19):12001–5.
Maltesen MJ, Bjerregaard S, Hovgaard L, Havelund S, van de Weert M. Quality by design – Spray drying of insulin intended for inhalation. Eur J Pharm Biopharm. 2008;70(3):828–38.
Yang M, Velaga S, Yamamoto H, Takeuchi H, Kawashima Y, Hovgaard L, et al. Characterisation of salmon calcitonin in spray-dried powder for inhalation. Effect of chitosan. Int J Pharm. 2007;331(2):176–81.
Maa YF, Hsu CC. Effect of high shear on proteins. Biotechnol Bioeng. 1996;51:458–65.
Maa YF, Prestrelski SJ. Biopharmaceutical powders particle formation and formulation considerations. Curr Pharm Biotechnol. 2000;1(3):283–302.
Kumar R, Palmieri Jr MJ. Points to consider when establishing drug product specifications for parenteral microspheres. AAPS J. 2010;12(1):27–32.
Park JH, Ye M, Yeo Y, Lee W-K, Paul C, Park K. Reservoir-Type Microcapsules Prepared by the Solvent Exchange Method Effect of Formulation Parameters on Microencapsulation of Lysozyme. Mol Pharm. 2006;3(2):135–43.
Xie J, Ng WJ, Lee LY, Wang CH. Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray. J Colloid Interface Sci. 2008;317(2):469–76.
Xie J, Wang CH. Encapsulation of proteins in biodegradable polymeric microparticles using electrospray in the Taylor cone-jet mode. Biotechnol Bioeng. 2007;97(5):1278–90.
Xu Y, Hanna MA. Electrospray encapsulation of water-soluble protein with polylactide. Effects of formulations on morphology, encapsulation efficiency and release profile of particles. Int J Pharm. 2006;320(1–2):30–6.
Ozeki T, Beppu S, Mizoe T, Takashima Y, Yuasa H, Okada H. Preparation of Polymeric Submicron Particle-Containing Microparticles Using a 4-Fluid Nozzle Spray Drier. Pharm Res. 2006;23(1):177–83.
Mizoe T, Beppu S, Ozeki T, Okada H. One-step preparation of drug-containing microparticles to enhance the dissolution and absorption of poorly water-soluble drugs using a 4-fluid nozzle spray drier. J Control Release. 2007;120(3):205–10.
Mizoe T, Ozeki T, Okada H. Preparation of drug nanoparticle-containing microparticles using a 4-fluid nozzle spray drier for oral, pulmonary, and injection dosage forms. J Control Release. 2007;122(1):10–5.
Ritesh M. Pabari, Tara Sunderland, Ramtoola Z. Investigation of a novel 3-fluid nozzle spray drying technology for the engineering of multifunctional layered microparticles. Expert Opin Drug Deliv. 2012;9(12):1463–74.
Legako J, Dunford NT. Effect of spray nozzle design on fish oil-whey protein microcapsule properties. J Food Sci. 2010;75(6):E394–400.
Kondo K, Niwa T, Danjo K. Preparation of sustained-release coated particles by novel microencapsulation method using three-fluid nozzle spray drying technique. Eur J Pharm Sci. 2014;51:11–9.
Cun D, Jensen DK, Maltesen MJ, Bunker M, Whiteside P, Scurr D, et al. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: Quality by design optimization and characterization. Eur J Pharm Biopharm. 2011;77(1):26–35.
O’Hagan DT, Jeffery H, Davis SS. The preparation and characterization of poly(lactide-co-glycolide) microcapsules III. Microparticlepolymer degradation rates and the in vitro release of a model protein. Int J Pharm. 1994;103:37–45.
Weers JG, Tarara TE, Clark AR. Design of fine particles for pulmonary drug delivery. Expert Opin Drug Deliv. 2007;4:297–313.
Aulton ME, Taylor KMG. Aulton’s Pharmaceutics: The design and manufacture of medicines: Churchill Livingstone, ELSEVIER; 2013.
Kang J, Schwendeman SP. Pore Closing and Opening in Biodegradable Polymers and Their Effect on the Controlled Release of Proteins. Mol Pharm. 2007;4(1):104–18.
Sax G, Winter G. Mechanistic studies on the release of lysozyme from twin-screw extruded lipid implants. J Control Release. 2012;163(2):187–94.
Yang Y, Li X, Qi M, Zhou S, Weng J. Release pattern and structural integrity of lysozyme encapsulated in core-sheath structured poly(DL-lactide) ultrafine fibers prepared by emulsion electrospinning. Eur J Pharm Biopharm. 2008;69(1):106–16.
Yeo Y, Chen AU, Basaran OA, Park K. Solvent exchange method: A novel microencapsulation technique using dual microdispensers. Pharm Res. 2004;21(8):1419–27.
Martin AN. Physical Pharmacy. Philadelphia: Lea & Febiger; 1993.
Yeo Y, Basaran OA, Park K. A new process for making reservoir-type microcapsules using ink-jet technology and interfacial phase separation. J Control Release. 2003;93(2):161–73.
Torza S, Mason SG. Three-phase interactions in shear and electrical fields. J Colloid Interface Sci. 1970;33:67–83.
Adler M, Unger M, Lee G. Surface composition of spray-dried particles of bovine serum albumin/trehalose/surfactant. Pharm Res. 2000;17(7):863–70.
Wan F, Bohr A, Maltesen MJ, Bjerregaard S, Foged C, Rantanen J, et al. Critical Solvent Properties Affecting the Particle Formation Process and Characteristics of Celecoxib-Loaded PLGA Microparticles via Spray-Drying. Pharm Res. 2013;30(4):1065–76.
Mei F, Chen DR. Investigation of compound jet electrospray: Particle encapsulation. Phys Fluids. 2007;19(10):103303.
Wulsten E, Kiekens F, van Dycke F, Voorspoels J, Lee G. Levitated single-droplet drying: case study with itraconazole dried in binary organic solvent mixtures. Int J Pharm. 2009;378(1–2):116–21.
Schiffter HA. Single droplet drying of proteins and protein formulations via acoustic levitation. PhD Thesis Friedrich-Alexander-University, Erlangen, Germany. 2005.
Vehring R, Foss W, Lechugaballesteros D. Particle formation in spray drying. J Aerosol Sci. 2007;38(7):728–46.
Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int J Pharm. 2011;415(1–2):34–52.
Wan F, Wu JX, Bohr A, Baldursdottir SG, Maltesen MJ, Bjerregaard S, et al. Impact of PLGA molecular behavior in the feed solution on the drug release kinetics of spray dried microparticles. Polymer. 2013;54(21):5920–7.
Acknowledgments and Disclosures
This work was funded by The Danish Council for Technology and Innovation via the Innovation Consortium NanoMorph (952320/2009), The Drug Research Academy and The Danish Agency for Science, Technology and Innovation. The authors would also like to thank Erik Wisaeus, Kenneth Brian Haugshøj and Pia Wahlberg (Danish Technological Institute) and for technical assistance with the SEM, FIB-SEM, and XPS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wan, F., Maltesen, M.J., Andersen, S.K. et al. One-Step Production of Protein-Loaded PLGA Microparticles via Spray Drying Using 3-Fluid Nozzle. Pharm Res 31, 1967–1977 (2014). https://doi.org/10.1007/s11095-014-1299-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1299-1