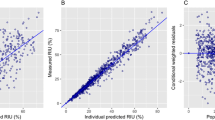
ABSTRACT
Purpose
To develop a systems pharmacology model based on hormone physiology and pharmacokinetic-pharmacodynamic concepts describing the impact of thyroperoxidase (TPO) inhibition on thyroid hormone homeostasis in the dog and to predict drug-induced changes in thyroid hormones in humans.
Methods
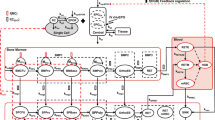
A population model was developed based on a simultaneous analysis of concentration-time data of T4, T3 and TSH in dogs following once daily oral dosing for up to 6-months of a myeloperoxidase inhibitor (MPO-IN1) with TPO inhibiting properties. The model consisted of linked turnover compartments for T4, T3 and TSH including a negative feedback from T4 on TSH concentrations.
Results
The model could well describe the concentration-time profiles of thyroid hormones in dog. Successful model validation was performed by predicting the hormone concentrations during 1-month administration of MPO-IN2 based on its in vitro dog TPO inhibition potency. Using human thyroid hormone turnover rates and TPO inhibitory potency, the human T4 and TSH concentrations upon MPO-IN1 treatment were predicted well.
Conclusions
The model provides a scientific framework for the prediction of drug induced effects on plasma thyroid hormones concentrations in humans via TPO inhibition based on results obtained in in vitro and animal studies.






Similar content being viewed by others
Abbreviations
- AUC0-24 :
-
Area under the plasma concentration-time curve from time 0–24 h after dosing
- C ss :
-
Steady state plasma concentration of the MPO inhibitor
- DIT:
-
Diiodotyrosine
- DRUG :
-
Function to describe the drug-induced inhibition of T4 production
- FEED1 :
-
Influence of T4 on TSH production
- FEED2 :
-
Influence of T4 on TSH turnover
- fr :
-
The fraction of T4 that undergoes peripheral conversion to T3
- Fraction :
-
The fraction of T3 converted from T4
- HPT:
-
Hypothalamic-pituitary-thyroid
- IC 50 :
-
Concentration which produces 50% of maximum inhibition of TPO
- I max :
-
Maximal inhibition of TPO production
- kin T3 :
-
Zero-order production rate of T3
- kin T4 :
-
Zero-order production of (the precursor of) T4
- kin TSH :
-
Zero-order production of (the precursor of) TSH
- k T3 :
-
First-order rate constant of elimination of T3
- k T4 :
-
First-order rate constant of elimination of T4
- k TSH :
-
First-order rate constant of elimination of TSH
- LC-MS/MS:
-
Liquid chromatography with mass spectrometry detection
- LLOQ:
-
Lower limit of quantification
- MIT:
-
Monoiodotyrosine
- MPO:
-
Myeloperoxidase
- MPO-IN1:
-
MPO inhibitor 1
- MPO-IN2:
-
MPO inhibitor 2
- n :
-
Number of transit compartments
- NF1 :
-
Slope factor of FEED1 relationship
- NF2 :
-
Slope factor of FEED2 relationship
- NF3 :
-
Slope factor in STIM function
- PKPD:
-
Pharmacokinetic-pharmacodynamic
- rT3 :
-
Non-active reverse T3
- STIM :
-
Function to describe TSH influencing the production of T4
- T3 :
-
Triiodothyronine
- T3,BL :
-
Baseline of T3 in plasma
- T4 :
-
Thyroxine
- T4,BL :
-
Baseline of T4 in plasma
- TPO:
-
Thyroperoxidase
- TRH:
-
Thyrotropin-releasing hormone
- TSH:
-
Thyroid stimulating hormone
- TSHBL :
-
Baseline of TSH in plasma
REFERENCES
Guyton AC, Hall JE. Textbook of medicial physiology. Eleventhth ed. Philadelphia: Elsevier Inc; 2006.
Zoeller RT, Tan SW, Tyl RW. General Background on the Hypothalamic-Pituitary-Thyroid (HPT) Axis. Crit Rev Toxicol 2007 01/01; 2012/08;37(1, 2):11–53.
Jameson JL, Weetman AP. Disorders of the thyroid gland. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine, 15th Edition: McGraw-Hill Companies, Inc; 2001. p. 2060–2064.
Fliers E, Alkemade A, Wiersinga WM, Swaab DF. Hypothalamic thyroid hormone feedback in health and disease. In: Kalsbeek, Fliers E, Hofman, Swaab, Van Someren, Buys, editors. Progress in Brain Research: Elsevier; 2006. p. 189–207.
Ridgway EC, Weintraub BD, Maloof F. Metabolic clearance and production rates of human thyrotropin. J Clin Invest. 1974;53(3):895–903.
Nussey S, Whitehead S. An integrated Approach. Oxford: Bios Scientific Publishers. 2001.
McGuire RA, Hays MT. A kinetic model of human thyroid hormones and their conversion products. J Clin Endocrinol Metab. 1981;53(4):852–62.
Belshaw BE, Barandes M, Becker DV, Berman M. A model of iodine kinetics in the dog. Endocrinology. 1974;95(4):1078–93.
Kaptein EM, Moore GE, Ferguson DC, Hoenig M. Thyroxine and triiodothyronine distribution and metabolism in thyroxine-replaced athyreotic dogs and normal humans. Am J Physiol. 1993;264(1 Pt 1):E90–E100.
van der Graaf PH, Benson N. Systems pharmacology: bridging systems biology and pharmacokinetics-pharmacodynamics (PKPD) in drug discovery and development. Pharm Res. 2011;28(7):1460–4.
Degon M, Chipkin SR, Hollot CV, Zoeller RT, Chait Y. A computational model of the human thyroid. Math Biosci. 2008;212(1):22–53.
Hatakeyama T, Yagi H. Computer simulation for hormones related to primary thyropathy. Biol Cybern. 1985;52(4):259–66.
Saratchandran P, Carson ER, Reeve J. An improved mathematical model of human thyroid hormone regulation. Clin Endocrinol (Oxf). 1976;5(5):473–83.
DiStefano 3rd JJ, Stear EB. On identification of hypothalamo-hypophysial control and feedback relationships with the thyroid gland. J Theor Biol. 1968;19(1):29–50.
Danziger L, Elmergreen G. Mathematical models of endocrine systems. Bull Math Biol. 1957;19(1):9–18.
Hays MT, Broome MR, Turrel JM. A multicompartmental model for iodide, thyroxine, and triiodothyronine metabolism in normal and spontaneously hyperthyroid cats. Endocrinology. 1988;122(6):2444–61.
McLanahan ED, Andersen ME, Fisher JW. A biologically based dose–response model for dietary iodide and the hypothalamic-pituitary-thyroid axis in the adult rat: evaluation of iodide deficiency. Toxicol Sci. 2008;102(2):241–53.
Merrill EA, Clewell RA, Robinson PJ, Jarabek AM, Gearhart JM, Sterner TR, et al. PBPK model for radioactive iodide and perchlorate kinetics and perchlorate-induced inhibition of iodide uptake in humans. Toxicol Sci. 2005;83(1):25–43.
Eisenberg M, Samuels M, DiStefano 3rd JJ. Extensions, validation, and clinical applications of a feedback control system simulator of the hypothalamo-pituitary-thyroid axis. Thyroid. 2008;18(10):1071–85.
Eisenberg M, Samuels M, DiStefano 3rd JJ. L-T4 bioequivalence and hormone replacement studies via feedback control simulations. Thyroid. 2006;16(12):1279–92.
Eisenberg MC, Santini F, Marsili A, Pinchera A, DiStefano 3rd JJ. TSH regulation dynamics in central and extreme primary hypothyroidism. Thyroid. 2010;20(11):1215–28.
Mukhopadhyay B, Bhattacharyya R. A mathematical model describing the thyroid-pituitary axis with time delays in hormone transportation. Appl Math. 2006;6:549–64.
Dietrich JW, Boehm BO. Equilibrium behaviour of feedback-coupled physiological saturation kinetics. Cybern Syst. 2006;1:269–74.
Dietrich JW, Tesche A, Pickardt CR, Mitzdorf U. Thyrotropic feedback control: evidence of an additional ultrashort feedback loop from fractal analysis. Cybern Syst 2004 06/01; 2012/08;35(4):315–331.
Kimura S, Ikeda-Saito M. Human myeloperoxidase and thyroid peroxidase, two enzymes with separate and distinct physiological functions, are evolutionarily related members of the same gene family. Proteins. 1988;3(2):113–20.
Malle E, Furtmüller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152(6):838–54.
Nilsson LB, Eklund G. Direct quantification in bioanalytical LC–MS/MS using internal calibration via analyte/stable isotope ratio. J Pharm Biomed Anal. 2007;43(3):1094–9.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26.
Ganjam VK, Wyckoff JT, Comerci CA, Ravis WR. Recrudescence of extra‐thyroidal tissue. T4 and T3 kinetics following thyroidectomy and effect of replacement therapy in the canine. Fed Proc 1980;39:947.
Kinlaw WB, Schwartz HL, Oppenheimer JH. Decreased serum triiodothyronine in starving rats is due primarily to diminished thyroidal secretion of thyroxine. J Clin Invest. 1985;75(4):1238–41.
Nicoloff JT, Low JC, Dussault JH, Fisher DA. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J Clin Invest. 1972;51(3):473–83.
Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National health and nutrition examination survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007;92(11):4236–40.
Liu Y, Liu B, Xie J, Liu YX. A new mathematical model of hypothalamo-pituitary-thyroid axis. Math Comput Model. 1994;19(9):81–90.
Li G, Liu B, Liu Y. A dynamical model of the pulsatile secretion of the hypothalamo-pituitary-thyroid axis. Biosystems. 1995;35(1):83–92.
Hays MT, McGuire RA. Distribution of subcutaneous thyroxine, triiodothyronine, and albumin in man: comparison with intravenous administration using a kinetic model. J Clin Endocrinol Metab. 1980;51(5):1112–7.
Kaplan MM. The role of thyroid hormone deiodination in the regulation of hypothalamo-pituitary function. Neuroendocrinology. 1984;38(3):254–60.
Acknowledgments AND DISCLOSURES
Håkan Eriksson, Anders Viberg, Olof Breuer, Bart Ploeger and Bert Peletier for valuable discussions. The authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekerot, P., Ferguson, D., Glämsta, EL. et al. Systems Pharmacology Modeling of Drug-Induced Modulation of Thyroid Hormones in Dogs and Translation to Human. Pharm Res 30, 1513–1524 (2013). https://doi.org/10.1007/s11095-013-0989-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-0989-4