ABSTRACT
Purpose
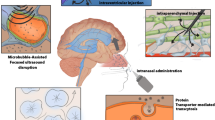
Delivery of therapeutic proteins across the blood-brain barrier (BBB) is severely limited by their size and biochemical properties. Here we showed that a 39-amino acid peptide derived from the rabies virus glycoprotein (RDP) was exploited as an efficient protein carrier for brain-targeting delivery.
Methods
Three proteins with different molecular weight and pI, β-galactosidase (β-Gal), luciferase (Luc) and brain-derived neurotrophic factor (BDNF), were fused to RDP and intravenously injected into the mice respectively. The slices of different tissues with X-Gal staining were used to examine whether RDP could deliver β-Gal targeted into the CNS. The time-course relationship of RDP-Luc was studied to confirm the transport efficiency of RDP. The neuroprotective function of RDP-BDNF was examined in mouse experimental stroke to explore the pharmacological effect of RDP fusion protein.
Results
The results showed that the fusion proteins rapidly and specific entered the nerve cells in 15 min, and the t1/2 was about 1 hr. Furthermore, RDP-BDNF fusion protein showed the neuroprotective properties in mouse experimental stroke including reduction of stroke volume and neural deficit.
Conclusions
RDP provides an effective approach for the targeted delivery of biological active proteins into the central nervous system.




Similar content being viewed by others
REFERENCES
Banks WA. Physiology and pathology of the blood-brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J Neurovirol. 1999;5(6):538–55.
Nathanson D, Mischel PS. Charting the course across the blood-brain barrier. J Clin Invest. 2011;121(1):31–3.
Pardridge WM. Biopharmaceutical drug targeting to the brain. J Drug Target. 2010;18(3):157–67.
Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104(1):29–45.
Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23(1):35–58.
Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci USA. 2007;104:7594–9.
Pardridge WM. Peptide drug delivery to the brain. New York: Raven; 1991.
Hammarlund-Udenaes M, Fridén M, Syvänen S, Gupta A. On the rate and extent of drug delivery to the brain. Pharm Res. 2008;25(8):1737–50.
Kilic U, Kilic E, Dietz GP, Bähr M. Intravenous TAT-GDNF is protective after focal cerebral ischemia in mice. Stroke. 2003;34(5):1304–10.
Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72.
Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57(4):637–51.
Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm Res. 2007;24(11):1977–92.
Grdisa M. The delivery of biologically active (therapeutic) peptides and proteins into cells. Curr Med Chem. 2011;18(9):1373–9.
Fu AL, Li Q, Dong ZH, Huang SJ, Wang YX, Sun MJ. Alternative therapy of Alzheimer’s disease via supplementation with choline acetyltransferase. Neurosci Lett. 2004;368(3):258–62.
Fu AL, Wu SP, Dong ZH, Sun MJ. A novel therapeutic approach to depression via supplement with tyrosine hydroxylase. Biochem Bioph Res Co. 2006;351(1):140–5.
Wu SP, Fu AL, Wang YX, Yu LP, Jia PY, Li Q, et al. A novel therapeutic approach to 6-OHDA-induced Parkinson’s disease in rats via supplementation of PTD-conjugated tyrosine hydroxylase. Biochem Bioph Res Co. 2006;346(1):1–6.
Martín I, Teixidó M, Giralt E. Building cell selectivity into CPP-Mediated strategies. Pharm. 2010;3:1456–90.
Toivonen JM, Oliván S, Osta R. Tetanus toxin C-fragment: the courier and the cure? Toxins. 2010;2:2622–44.
Tuffereau C, Benejean J, Alfonso AR, Flamand A, Fishman AC. Neuronal cell surface molecules mediate specific binding to rabies virus glycoprotein expressed by a recombinant baculovirus on the surfaces of lepidopteran cells. J Virol. 1998;72:1085–91.
Tang Q, Orciari LA, Rupprechti CE, Zhao XQ, Li ZG, Yang WS. Sequencing and position analysis of the glycoprotein gene of four Chinese rabies viruses. Virol Sin. 2000;15(1):22–33.
Tuffereau C, Leblois H, Bénéjean J, Coulon P, Lafay F, Flamand A. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology. 1989;172(1):206–12.
Xiang L, Zhou R, Fu A, Xu X, Huang Y, Hu C. Targeted delivery of large fusion protein into hippocampal neurons by systemic administration. J Drug Target. 2011;19:632–6.
Fu A, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Neuroprotection in stroke in the mouse with intravenous erythropoietin-Trojan horse fusion protein. Brain Res. 2011;1369:203–7.
Cai SR, Xu G, Becker-Hapak M, Ma M, Dowdy SF, McLeod HL. The kinetics and tissue distribution of protein transduction in mice. Eur J Pharm Sci. 2006;27(4):311–9.
Horsburgh MJ, Ingham E, Foster SJ. In Staphylococcus aureus, fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J Bacteriol. 2001;183:468–75.
Masahira N, Ding L, Takebayashi H, Shimizu K, Ikenaka K, Ono K. Improved preservation of X-gal reaction product for electron microscopy using hydroxypropyl methacrylate. Neurosci Lett. 2005;374:17–20.
Bloquel C, Trollet C, Pradines E, Seguin J, Scherman D, Bureau MF. Optical imaging of luminescence for in vivo quantification of gene electrotransfer in mouse muscle and knee. BMC Biotechnol. 2006;6:16.
Montag C, Schoene-Bake JC, Faber J, Reuter M, Weber B. Genetic variation on the BDNF gene is not associated with differences in white matter tracts in healthy humans measured by tract-based spatial statistics. Genes Brain Behav. 2010;9(8):886–91.
Zhou JP, Feng ZG, Yuan BL, Yu SZ, Li Q, Qu HY, et al. Transduced PTD-BDNF fusion protein protects against beta amyloid peptide-induced learning and memory deficits in mice. Brain Res. 2008;1191:12–9.
Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet. 2001;10(19):2109–21.
Benmansour A, Leblois H, Coulon C, Tuffereau C, Gaudin Y, Flamand A, et al. Antigenicity of rabies virus glycoprotein. J Virol. 1991;65:4198–203.
Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31(3):349–52.
Resende RR, Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal. 2009;7:20.
Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46.
Brüggmann D, Lips KS, Pfeil U, Haberberger RV, Kummer W. Multiple nicotinic acetylcholine receptor alpha-subunits are expressed in the arterial system of the rat. Histochem Cell Biol. 2002;118(6):441–7.
Brüggmann D, Lips KS, Pfeil U, Haberberger RV, Kummer W. Rat arteries contain multiple nicotinic acetylcholine receptor alpha-subunits. Life Sci. 2003;72(18–19):2095–9.
Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43.
do Hwang W, Son S, Jang J, Youn H, Lee S, Lee D, et al. brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials. 2011;32(21):4968–75.
Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30:4195–202.
Laffray S, Tan K, Dulluc J, Bouali-Benazzouz R, Calver AR, Nagy F, et al. Dissociation and trafficking of rat GABAB receptor heterodimer upon chronic capsaicin stimulation. Eur J Neurosci. 2007;25(5):1402–16.
ACKNOWLEDGMENTS & DISCLOSURES
This work is supported by the grants from the Natural Science Foundation of China (31072098), the Doctoral Fund of Ministry of Education of China (20090182120017) and the Fundamental Research Funds for the Central Universities of China (XDGK2009C174).
We are grateful to Miss Lixin Xiang for pcDNA/Lac Z plasmid, to Dr. Ying Bin Yang for pVAX/Luc plasmid, and to Dr. Jian Ping Zhou for pGST-4T/BDNF plasmid.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, A., Wang, Y., Zhan, L. et al. Targeted Delivery of Proteins into the Central Nervous System Mediated by Rabies Virus Glycoprotein-Derived Peptide. Pharm Res 29, 1562–1569 (2012). https://doi.org/10.1007/s11095-012-0667-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0667-y