ABSTRACT
Purpose
Magnetic nanoparticles (MNPs) and magnets can be used to enhance gene transfer or cell attachment but gene or cell delivery to confined areas has not been addressed. We therefore searched for an optimal method to simulate and perform local gene targeting and cell delivery in vitro.
Methods
Localized gene transfer or cell positioning was achieved using permanent magnets with newly designed soft iron tips and MNP/lentivirus complexes or MNP-loaded cells, respectively. Their distribution was simulated with a mathematical model calculating magnetic flux density gradients and particle trajectories.
Results
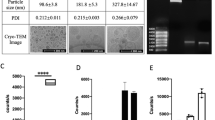
Soft iron tips generated strong confined magnetic fields and could be reliably used for local (~500 μm diameter) gene targeting and positioning of bone marrow cells or cardiomyocytes. The calculated distribution of MNP/lentivirus complexes and MNP-loaded cells concurred very well with the experimental results of local gene expression and cell attachment, respectively.
Conclusion
MNP-based gene targeting and cell positioning can be reliably performed in vitro using magnetic soft iron tips, and computer simulations are effective methods to predict and optimize experimental results.







Similar content being viewed by others
Abbreviations
- BMC:
-
bone marrow cells
- GFP:
-
green fluorescent protein
- MNP:
-
magnetic nanoparticle
- MEA:
-
microelectrode array
REFERENCES
Krishnan KM. Biomedical nanomagnetics: a spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans Magn. 2010;46(7):2523–58.
Lübbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res. 2001;95(2):200–6.
Alexiou C, Jurgons R, Schmid RJ, Bergemann C, Henke J, Erhardt W, et al. Magnetic drug targeting–biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatment. J Drug Target. 2003;11(3):139–49.
Ally J, Martin B, Khamesee MB, Roa W, Amirfazli A. Magnetic targeting of aerosol particles for cancer therapy. J Magn Magn Mater. 2005;293(1):442–9.
Dames P, Gleich B, Flemmer A, Hajek K, Seidl N, Wiekhorst F, et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat Nanotechnol. 2007;2(8):495–9.
Plank C, Schillinger U, Scherer F, Bergemann C, Remy JS, Krotz F, et al. The magnetofection method: using magnetic force to enhance gene delivery. Biol Chem. 2003;384(5):737–47.
Al-Deen FN, Ho J, Selomulya C, Ma C, Coppel R. Superparamagnetic nanoparticles for effective delivery of malaria DNA vaccine. Langmuir. 2011;27(7):3703–12.
Mykhaylyk O, Zelphati O, Rosenecker J, Plank C. siRNA delivery by magnetofection. Curr Opin Mol Ther. 2008;10(5):493–505.
Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9(2):102–9.
Hofmann A, Wenzel D, Becher UM, Freitag DF, Klein AM, Eberbeck D, et al. Combined targeting of lentiviral vectors and positioning of transduced cells by magnetic nanoparticles. Proc Natl Acad Sci U S A. 2009;106(1):44–9.
Haim H, Steiner I, Panet A. Synchronized infection of cell cultures by magnetically controlled virus. J Virol. 2005;79(1):622–5.
Gleich B, Weyh T, Wolf B. Magnetic drug targeting: an analytical model for the influence of blood properties on particle trajectories. Applied Rheology 2008;18(5).
Babinec P, Krafcik A, Babincova M, Rosenecker J. Dynamics of magnetic particles in cylindrical Halbach array: implications for magnetic cell separation and drug targeting. Med Biol Eng Comput. 2010;48(8):745–53.
Krafcik A, Babinec P, Babincova M. Feasibility of subcutaneously implanted magnetic microarrays for site specific drug and gene targeting. J Eng Sci Tech Rev. 2010;3(1):53–7.
Gleich B, Hellwig N, Bridell H, Jurgons R, Seliger C, Alexiou C, et al. Design and evaluation of magnetic fields for nanoparticle drug targeting in cancer. IEEE Trans Nanotechnol. 2007;6(2):164–70.
Sharma MM, Chamoun H, Sarma DSHS, Schechter RS. Factors controlling the hydrodynamic detachment of particles from surfaces. J Colloid Interface Sci. 1992;149(1):121–34.
Cornell U, Schwertmann U. The Iron Oxides. Weinheim, Germany: VCH Publishing Group; 1996.
Hafeli UO, Lobedann MA, Steingroewer J, Moore LR, Riffle J. Optical method for measurement of magnetophoretic mobility of individual magnetic microspheres in defined magnetic field. J Magn Magn Mater. 2005;293(1):224–39.
Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95(6):2979–84.
Trueck C, Zimmermann K, Mykhaylyk O, Anton M, Vosen S, Wenzel D, et al. Optimization of magnetic nanoparticles assisted lentiviral gene transfer. Pharm Res. 2011. doi:10.1007/s11095-011-0660-x.
Mykhaylyk O, Sanchez-Antequera Y, Vlaskou D, Hammerschmid E, Anton M, Zelphati O, et al. Liposomal magnetofection. Meth Mol Biol. 2010;605:487–525.
Mykhaylyk O, Sobisch T, Almstätter I, Sanchez-Antequera Y, Brandt S, Anton M, et al. Silica-iron oxide magnetic nanoparticles modified for gene delivery. A search for optimum and quantitative criteria. Pharm Res. 2011. doi:10.1007/s11095-011-0661-9.
Pfeifer A, Hofmann A. Lentiviral transgenesis. Meth Mol Biol. 2009;530:391–405.
Becher UM, Breitbach M, Sasse P, Garbe S, van der Ven PF, Furst DO, et al. Enrichment and terminal differentiation of striated muscle progenitors in vitro. Exp Cell Res. 2009;315(16):2741–51.
Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110(4):1362–9.
Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203(10):2315–27.
Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK, et al. Optogenetic control of heart muscle in vitro and in vivo. Nat Methods. 2010;7(11):897–900.
Pislaru SV, Harbuzariu A, Gulati R, Witt T, Sandhu NP, Simari RD, et al. Magnetically targeted endothelial cell localization in stented vessels. J Am Coll Cardiol. 2006;48(9):1839–45.
Murry CE, Field LJ, Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112(20):3174–83.
Malan D, Friedrichs S, Fleischmann BK, Sasse P. Cardiomyocytes Obtained From Induced Pluripotent Stem Cells With Long-QT Syndrome 3 Recapitulate Typical Disease-Specific Features In Vitro. Circ Res 2011;109(8):841–7.
Weber W, Lienhart C, Baba MDE, Grass RN, Kohler T, Muller R, et al. Magnet-guided transduction of mammalian cells and mice using engineered magnetic lentiviral particles. J Biotechnol. 2009;141(3–4):118–22.
Furlani EJ, Furlani EP. A model for predicting magnetic targeting of multifunctional particles in the microvasculature. J Magn Magn Mater. 2007;312(1):187–93.
David AE, Cole AJ, Chertok B, Park YS, Yang VC. A combined theoretical and in vitro modeling approach for predicting the magnetic capture and retention of magnetic nanoparticles in vivo. J Control Release. 2011;152(1):67–75.
Shimizu K, Ito A, Lee JK, Yoshida T, Miwa K, Ishiguro H, et al. Construction of multi-layered cardiomyocyte sheets using magnetite nanoparticles and magnetic force. Biotechnol Bioeng. 2007;96(4):803–9.
Akiyama H, Ito A, Sato M, Kawabe Y, Kamihira M. Construction of cardiac tissue rings using a magnetic tissue fabrication technique. Int J Mol Sci. 2010;11(8):2910–20.
Chorny M, Fishbein I, Yellen BB, Alferiev IS, Bakay M, Ganta S, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci U S A. 2010;107(18):8346–51.
Riegler J, Wells JA, Kyrtatos PG, Price AN, Pankhurst QA, Lythgoe MF. Targeted magnetic delivery and tracking of cells using a magnetic resonance imaging system. Biomaterials. 2010;31(20):5366–71.
ACKNOWLEDGMENTS & DISCLOSURES
We thank O. Mykhaylyk and C. Plank, Institute for Experimental Oncology and Therapy Research, Technische Universitaet Muenchen, Germany for providing the SO-Mag5 MNP, K. Zimmermann and A. Pfeifer, Institute of Pharmacology and Toxicology, University of Bonn, Germany for providing the cytomegalovirus-GFP lentivirus, W. C. Claycomb, New Orleans, USA for providing the HL-1 cell line, and K. Granitza and the machine shop of the Institute of Physiology, University Bonn for machining the soft iron tips.
This work was supported by grants of the German Research Foundation within the DFG Research Unit 917 “Nanoparticle-based targeting of gene- and cell-based therapies.”
Author information
Authors and Affiliations
Corresponding authors
Additional information
Carsten Kilgus and Alexandra Heidsieck contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Kilgus, C., Heidsieck, A., Ottersbach, A. et al. Local Gene Targeting and Cell Positioning Using Magnetic Nanoparticles and Magnetic Tips: Comparison of Mathematical Simulations with Experiments. Pharm Res 29, 1380–1391 (2012). https://doi.org/10.1007/s11095-011-0647-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0647-7