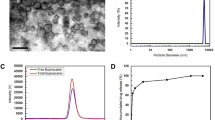
ABSTRACT
Purpose
To reduce formulation viscosity of bupivacaine/poly(DL lactic acid co castor oil) 3:7 without increasing bupivacaine release rates.
Methods
Poly(DL lactic acid) 3:7 was synthesized and bupivacaine formulation prepared by mixing with additives ricinoleic acid or castor oil. In vitro release measurements identified optimum formulation. Anesthetized ICR mice were injected around left sciatic nerve using nerve stimulator with 0.1 mL of formulation. Animals received 10% bupivacaine-polymer formulation with 10% castor oil (p(DLLA:CO)3:7–10% bupi-10% CO) or 15% bupivacaine-polymer with 10% castor oil (p(DLLA:CO)3:7–15% bupi-10% CO). Sensory and motor block were measured.
Results
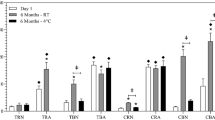
Viscosity of 10% and 15% bupivacaine-p(DLLA:CO)3:7 formulations was reduced using hydrophobic additives; however, castor oil reduced bupivacaine release rates and eliminated burst effect. Less than 10% of the incorporated bupivacaine was released during 6 h, and less than 25% released in 24 h in vitro. In vivo formulation injection resulted in a 24 h motor block and a sensory block lasting at least 72 h.
Conclusions
Incorporation of hydrophobic low-viscosity additive reduced viscosity in addition to burst release effects. Bupivacaine-polymer formulation with castor oil additive demonstrated prolonged sensory analgesia in vivo, with reduced duration of motor block.







Similar content being viewed by others
REFERENCES
Rawal N. Patient-controlled regional analgesia (PCRA) at home: controlled comparison between bupivacaine and ropivacaine brachial plexus analgesia. Anesthesiology. 2002;96:1290–6. Journal Article.
Calvey TN. Principles and practice of pharmacology for anaesthetists. 3rd ed. Oxford: Blackwell Science Ltd; 1997. p. 284–300. Chapter.
Hebl JR, Dilger JA, Byer DE, Kopp SL, Stevens SR, Pagnano MW, et al. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510–7. Journal Article.
Xu YX, Hanna MA. Preparation and properties of biodegradable foams from starch acetate and poly(tetramethylene adipate-co-terephthalate). Carbohydr Polym. 2005;59:521–9. Journal Article.
Masters DB, Berde CB, Dutta S, Turek T, Langer R. Sustained local anesthetic release from bioerodible polymer matrices: a potential method for prolonged regional anesthesia. Pharm Res. 1993;10:1527–32. Journal Article.
Pietkiewicz J, Sznitowska M, Placzek M. The expulsion of lipophilic drugs from the cores of solid lipid microspheres in diluted suspensions and in concentrates. Int J Pharm. 2006;310:64–71. Journal Article.
Colombo G, Langer R, Kohane DS. Effect of excipient composition on the biocompatibility of bupivacaine-containing microparticles at the sciatic nerve. J Biomed Mater Res A. 2004;68:651–9. Journal Article.
Curley J, Castillo J, Hotz J, Uezono M, Hernandez S, Lim JO, et al. Prolonged regional nerve blockade. Injectable biodegradable bupivacaine/polyester microspheres. Anesthesiology. 1996;84:1401–10. Journal Article.
Chen PC, Kohane DS, Park YJ, Bartlett RH, Langer R, Yang VC. Injectable microparticle-gel system for prolonged and localized lidocaine release. II. In vivo anesthetic effects. J Biomed Mater Res A. 2004;70(459):466. Journal Article.
Boogaerts J, Declercq A, Lafont N, Benameur H, Akodad EM, Dupont JC, et al. Toxicity of bupivacaine encapsulated into liposomes and injected intravenously: comparison with plain solutions. Anesth Analg. 1993;76:553–5. Journal Article.
Malinovsky JM, Le Corre P, Meunier JF, Chevanne F, Pinaud M, Leverge R, et al. A dose-response study of epidural liposomal bupivacaine in rabbits. J Contr Release. 1999;60:111–9. Journal Article.
Sokolsky-Papkov M, Golovanevski L, Domb A, Weiniger C. Prolonged local anesthetic action through slow release from poly(lactic acid co castor oil). Pharm Res. 2009;26:32–9. Journal Article.
Sokolsky-Papkov M, Golovanevski L, Domb A, Weiniger C. Poly(DL: lactic acid-castor oil) 3:7-bupivacaine formulation: reducing burst effect prolongs efficacy in vivo. J Pharm Sci. 2010;99:2732–8. Journal Article.
Shikanov A, Domb AJ, Weiniger CF. Long acting local anesthetic-polymer formulation to prolong the effect of analgesia. J Contr Release. 2007;117:97–103. Journal Article.
Grant GJ, Barenholz Y, Piskoun B, Bansinath M, Turndorf H, Bolotin EM. DRV liposomal bupivacaine: preparation, characterization, and in vivo evaluation in mice. Pharm Res. 2001;18:336–43. Journal Article.
Shikanov A, Vaisman B, Krasko MY, Nyska A, Domb AJ. Poly(sebacic acid-co-ricinoleic acid) biodegradable carrier for paclitaxel: in vitro release and in vivo toxicity. J Biomed Mater Res A. 2004;69A:47–54. Journal Article.
Larsen SW, Jessen MNB, Ostergaard J, Larsen C. Assessment of drug release from oil depot formulations using an in vitro model—potential applicability in accelerated release testing. Drug Dev Ind Pharm. 2008;34:297–304. Journal Article.
Vaisman B, Shikanov A, Domb AJ. The isolation of ricinoleic acid from castor oil by salt-solubility-based fractionation for the biopharmaceutical applications. J Am Oil Chem Soc. 2008;85:169–84. Journal Article.
Validation of analytical procedures: methodology proceedings of the International Conference on Harmonization (ICH) Topic Q2B (1996). http://www.scribd.com/doc/4058930/Analytical-Methods-A-Statistical-Perspective-on-the-ICH-Q2A-and-Q2B-Guidelines-for-Validation-of-Analytical-Methods
Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal Nociception in Cutaneous Hyperalgesia. Pain. 1988;32:77–88. Journal Article.
Shikanov A, Ezra A, Domb AJ. Poly(sebacic acid-co-ricinoleic acid) biodegradable carrier for paclitaxel-effect of additives. J Contr Release. 2005;105:52–67. Journal Article.
Winternitz CI, Jackson JK, Oktaba AM, Burt HM. Development of a polymeric surgical paste formulation for taxol. Pharm Res. 1996;13:368–75. Journal Article.
Dordunoo SK, Oktaba AMC, Hunter W, Min W, Cruz T, Burt HM. Release of taxol from poly(epsilon-caprolactone) pastes: effect of water-soluble additives. J Contr Release. 1997;44:87–94. Journal Article.
Song CX, Labhasetwar V, Levy RJ. Controlled release of U-86983 from double-layer biodegradable matrices: effect of additives on release mechanism and kinetics. J Contr Release. 1997;45:177–92. Journal Article.
Chang CM, Bodmeier R. Effect of dissolution media and additives on the drug release from cubic phase delivery systems. J Contr Release. 1997;46:215–22. Journal Article.
Jeong B, Bae YH, Kim SW. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J Contr Release. 2000;63:155–63. Journal Article.
Shively ML, Coonts BA, Renner WD, Southard J, Bennett AT. Physicochemical characterization of a polymeric injectable implant delivery system. J Contr Release. 1995;33:237–43. Journal Article.
Larsen DB, Joergensen S, Olsen NV, Hansen SH, Larsen C. In vivo release of bupivacaine from subcutaneously administered oily solution. Comparison with in vitro release. J Contr Release. 2002;81:145–54. Journal Article.
Larsen DB, Parshad H, Fredholt K, Larsen C. Characteristics of drug substances in oily solutions. Drug release rate, partitioning and solubility. Int J Pharm. 2002;232:107–17. Journal Article.
Kranz H, Yilmaz E, Brazeau GA, Bodmeier R. In vitro and in vivo drug release from a novel in situ forming drug delivery system. Pharm Res. 2008;25:1347–54. Journal Article.
Kranz H, Bodmeier R. Structure formation and characterization of injectable drug loaded biodegradable devices: in situ implants versus in situ microparticles. Eur J Pharm Sci. 2008;34:164–72. Journal Article.
Krasko MY, Shikanov A, Ezra A, Domb AJ. Poly(ester anhydride)s prepared by the insertion of ricinoleic acid into poly(sebacic acid). J Polymer Sci A-Polymer Chem. 2003;41:1059–69. Journal Article.
Slivniak R, Ezra A, Domb AJ. Hydrolytic degradation and drug release of ricinoleic acid-lactic acid copolyesters. Pharm Res. 2006;23:1306–12. Journal Article.
ACKNOWLEDGMENTS & DISCLOSURES
This research was supported in part by grant no. 5868 from the Chief Scientist Office of the Ministry of Health, Israel, by the Legacy Heritage Science Initiative Program of the Israel Science Foundation (1637/10) and a physician research grant from the Hadassah Hebrew University Medical Center
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sokolsky-Papkov, M., Golovanevski, L., Domb, A.J. et al. Long-Acting Poly(DL:Lactic Acid-Castor Oil) 3:7-Bupivacaine Formulation: Effect of Hydrophobic Additives. Pharm Res 28, 3265–3273 (2011). https://doi.org/10.1007/s11095-011-0497-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0497-3