Abstract
Purpose
In the present study, we have evaluated the pharmacokinetics and the in vivo prostate chemopreventive activity of broccoli sprouts.
Methods
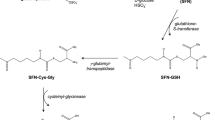
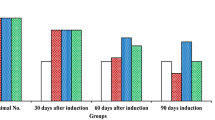
The in vivo pharmacokinetic profiles of sulforaphane (SFN) and SFN- glutathione (GSH) conjugate in rats after oral administration of 200 mg and 500 mg broccoli sprouts were analyzed. Next, 8-week old TRAMP mice were fed with dietary broccoli sprouts at two dosages (60 and 240 mg/mouse/day) for 16 weeks, and the mice were sacrificed to examine the pharmacodynamic response on prostate tumor and some biomarkers.
Results
SFN was readily released and conjugated with GSH in the rats after oral administration of broccoli sprouts. TRAMP mice fed with 240 mg broccoli sprouts/mouse/day exhibited a significant retardation of prostate tumor growth. Western blot analysis revealed that the expression levels of Nrf2, HO-1, cleaved-Caspase-3, cleaved-PARP and Bax proteins were increased, but that of Keap1 and Bcl-XL proteins were decreased. In addition, the phosphorylation and/or the expression level of Akt and its downstream kinase and target proteins, e.g. mTOR, 4E-BP1 and cyclin D1, were reduced.
Conclusions
Our findings indicate that broccoli sprouts can serve as a good dietary source of SFN in vivo and that they have significant inhibitory effects on prostate tumorigenesis.





Similar content being viewed by others
References
Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99.
Eastham JA. Prostate-specific antigen doubling time as a prognostic marker in prostate cancer. Nat Clin Pract Urol. 2005;2:482–91.
Klein EA. Can prostate cancer be prevented? Nat Clin Pract Urol. 2005;2:24–31.
Goetzland MA, Holzbeierlein JM. Finasteride as a chemopreventive agent in prostate cancer: impact of the PCPT on urologic practice. Nat Clin Pract Urol. 2006;3:422–9.
Kristaland AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9.
Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–90.
Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–403.
Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555:191–202.
Myzakand MC, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr Drug Targets. 2006;7:443–52.
Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–72.
Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–13.
Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem. 2005;343:93–9.
Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–9.
Barve A, Khor TO, Hao X, Keum YS, Yang CS, Reddy B, et al. Murine prostate cancer inhibition by dietary phytochemicals—curcumin and phenyethylisothiocyanate. Pharm Res. 2008;25:2181–9.
Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–9.
T.W. Kensler, N. Wakabayashi, and S. Biswal. Cell Survival Responses to Environmental Stresses Via the Keap1-Nrf2-ARE Pathway. Annu Rev Pharmacol Toxicol (2006).
Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106.
Shen G, Jeong WS, Hu R, Kong AN. Regulation of Nrf2, NF-kappaB, and AP-1 signaling pathways by chemopreventive agents. Antioxid Redox Signal. 2005;7:1648–63.
Hanahanand D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Danialand NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19.
Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–22.
Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34.
Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase- 3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511.
West LG, Meyer KA, Balch BA, Rossi FJ, Schultz MR, Haas GW. Glucoraphanin and 4-hydroxyglucobrassicin contents in seeds of 59 cultivars of broccoli, raab, kohlrabi, radish, cauliflower, brussels sprouts, kale, and cabbage. J Agric Food Chem. 2004;52:916–26.
Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937–44.
Lamband DJ, Zhang L. Challenges in prostate cancer research: animal models for nutritional studies of chemoprevention and disease progression. J Nutr. 2005;135:3009S–15.
Klein RD. The use of genetically engineered mouse models of prostate cancer for nutrition and cancer chemoprevention research. Mutat Res. 2005;576:111–19.
Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I- induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22.
Narayanan BA, Narayanan NK, Pittman B, Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clin Cancer Res. 2004;10:7727–37.
Gupta S, Adhami VM, Subbarayan M, MacLennan GT, Lewin JS, Hafeli UO, et al. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–43.
Garcia GE, Wisniewski HG, Lucia MS, Arevalo N, Slaga TJ, Kraft SL, et al. 2-Methoxyestradiol inhibits prostate tumor development in transgenic adenocarcinoma of mouse prostate: role of tumor necrosis factor-alpha-stimulated gene 6. Clin Cancer Res. 2006;12:980–8.
Raina K, Blouin MJ, Singh RP, Majeed N, Deep G, Varghese L, et al. Dietary feeding of silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2007;67:11083–91.
Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–25.
Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase- dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11:2670–9.
Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–25. Epub 2009 Feb 2117.
Chen ML, Xu PZ, Peng XD, Chen WS, Guzman G, Yang X, et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20:1569–74.
Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–7.
Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2006;103:14477–82.
Acknowledgement
We thank Mr. Richard Jarrell for providing us with the broccoli sprouts. We thank all the members in Dr. Ah-Ng “Tony” Kong’s lab for the help in discussion and preparation of this manuscript. This work was supported in part by the National Institute of Health Grant R01-CA118947 and Institutional Funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keum, YS., Oo Khor, T., Lin, W. et al. Pharmacokinetics and Pharmacodynamics of Broccoli Sprouts on the Suppression of Prostate Cancer in Transgenic Adenocarcinoma of Mouse Prostate (TRAMP) Mice: Implication of Induction of Nrf2, HO-1 and Apoptosis and the Suppression of Akt-dependent Kinase Pathway. Pharm Res 26, 2324–2331 (2009). https://doi.org/10.1007/s11095-009-9948-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9948-5